International Journal of
eISSN: 2381-1803


Review Article Volume 12 Issue 4
Department of Zoology, Mizoram University, India
Correspondence: Ganesh Chandra Jagetia, Department of Zoology, Mizoram University, India
Received: August 05, 2019 | Published: August 27, 2019
Citation: Jagetia GC, Zairempuii. Detection of free radical scavenging activity of dhaura, anogeissus acuminata (roxb) wall ex bedd in vitro. Int J Complement Alt Med. 2019;12(4):141-147. DOI: 10.15406/ijcam.2019.12.00464
The free radical inhibitory activity of Dhaura, Anogeissus acuminata extracted in chloroform, ethanol and water was investigated in vitro. The DPPH inhibition by the different stem extract depended on the concentration and the ethanol extract was found to be the most potent as the lower concentration of it was most effective in scavenging different free radicals. The analysis of superoxide radical scavenging activity showed that the aqueous extract was most effective when compared to chloroform and ethanol extracts. The different extracts of Dhaura also inhibited the generation of nitric oxide radical depending on its concentration and maximum effect was observed at 200μg/ml. Our study indicates that free radical scavenging activity of Dhaura affirms the medicinal use by the practitioners of folklore medicine.
Keywords: anogeissus acuminata, DPPH, superoxide, nitric oxide, endoplasmic reticulum, peroxisomes, mitochondria
The free radical generation is a universal phenomenon in eukaryotes utilizing oxygen for their energy requirements. The endoplasmic reticulum, peroxisomes, mitochondria, and lysosomes are the major sites of free radical generation in a cell.1–3 The free radicals are also generated by redox cycling of xenobiotics, exposure to ionizing radiations, radiofrequency and microwave radiations, visible light and UV radiation, many chemicals, oxygen– the most essential element of life, endogenous compounds and photosensitizing drugs.4–8 These free radicals trigger oxidative stress, which is one of the most important events in disease process and pathophysiology. It is not surprising that all most all diseases can be linked to triggering of oxidative stress and hence free radical formation in humans.9–11 Several synthetic drugs have been used to reduce oxidative stress however, they induce adverse toxic side effects.12,13 The use of plant derived or herbal products may be useful as antioxidants as they may not exert toxic adverse effects due to their biologic origin and composite nature.
Dhaura (Hindi) or Anogeissus acuminate- family Combretaceae grows up to a height of 40meters in semi–deciduous forests in India, Cambodia, Bangladesh, Laos, Myanmar, Arabian Peninsula, Thailand, Vietnam, and Africa.14,15 It is found in tropical and semideciduous regions of India. It has been used as a medicine in the Indian system of medicine, the Ayurveda. The Ayurveda describes it as an acrid, alexipharmic, bitter, astringent, cooling, and vulnerary. The roots of Dhaura are used for the treatment of asthma, dysentery, fatigue, leprosy, burning sensations, vaginal and uterine complaints, inflammations, leukoderma and blood diseases. The decoction prepared by boiling its roots in water reduces toothache after gargling.15 The amoebic dysentery, diarrhea, bleeding piles and urinary infections can also be treated by using Dhaura.15,16 Dhaura is utilized to treat skin disorders, neurological problems, general weakness, bronchitis, impotence, enlargement of spleen cold, dysuria, cough, excessive perspiration, cholera, urinary disorders, snake bite and in scorpion sting.15 The gum produced by Dhaura is called Ghatti or Indian gum and is used as a tonic after delivery.15,16 The preclinical studies have shown the alleviation of alloxan–induced diabetes in rats.17. The hydroalcoholic extracts of leaves and stem bark of Dhaura has been reported to be antioxidant and hepatoprotective in Wistar rats.18 The present study was undertaken to estimate the antioxidant activity of different stem bark extracts of Dhaura i.e. Anogeissus acuminata in vitro.
Chemicals
Analytical grade chemicals and MilliQ (18 Ω) water were used for entire analyses. Dimethylsulfoxide (DMSO), 1,1–Diphenyl–2–picrythydrazyl (DPPH), nitrobluetetrazolium (NBT), ethylenediaminetetraacetic acid (EDTA), trichloroacetic acid (TCA), sodium nitroprusside, sulfanilamide, N–(1–Naphthyl)ethylenediamine dihydrochloride (NED), and orthophosphoric acid were procured from Sigma Aldrich Chemicals co, Kolkata, India. Methanol, petroleum ether, chloroform, ethanol, sodium carbonate, sodium hydroxide, sodium chloride, potassium chloride, potassium nitrite, disodium hydrogen phosphate (anhydrous), potassium dihydrogen phosphate, aluminum chloride, ferrous ammonium sulphate, ammonium acetate, glacial acetic acid and acetyl acetone were procured from Merck India Ltd., Mumbai.
Preparation of extracts
Dhaura or Anogeissus acuminata belongs to family: Combretaceae. It is also known as button tree, Anogeissus pendula Edgew. Conocarpus acuminatus Roxb. ex DC. It was identified by the Department of Horticulture Aromatic and Medicinal Plants, Mizoram University, Aizawl, India. The mature non–infected stem bark of Dhaura was removed from the plant from the Mizoram University campus during the months of September to December and cleaned by washing thoroughly in water so as to remove the dust and other unnecessary extraneous material. The washed stem bark and shade dried cut into small pieces and ground into powder in an electrical grinder. The dried powder of Dhaura was sequentially extracted in petroleum ether, chloroform, ethanol and water using Soxhlet apparatus.
Free radical scavenging estimation
DPPH free radical scavenging activity: The DPPH inhibitory activity of chloroform, ethanol and water extracts were estimated as described earlier.19 with minor modifications. One ml of 0.1mM DPPH in methanol was added to the different concentrations of various extracts of Dhaura (0.5ml each). After mixing thoroughly, the mixture was allowed to stand in the dark for 30min and the absorbance was measured at 517nm using a UV–VIS spectrophotometer (SW 3.5.1.0. Biospectrometer, Eppendorf India Ltd., Chennai). The methanol was used for the baseline correction. The results have been compared with that of the control prepared as described above but without sample. DPPH radical scavenging activity has been expressed as a percentage and was calculated using the following formula:
Where is the absorbance of the test sample and is the absorbance of the control.
Superoxide anion scavenging activity: Superoxide scavenging activity was detected by the method of Hyland et al.20 The reaction mixture contained 0.2ml of NBT (1 mg/ml in DMSO), and 0.06ml different doses of different extracts and standard in 2ml of alkaline DMSO (1ml DMSO containing 5mMNaOH in 0.1ml H2O). Superoxide free radicals formed by alkaline DMSO react with NBT to produce coloured diformazan. The blank consisted of pure DMSO instead of alkaline DMSO. The absorbance was read at 560nm using UV–VIS spectrophotometer. The percentage inhibition of superoxide anion generation was calculated using the following formula.
Where is the absorbance of the control and is the absorbance of different extracts of Anogeissus acuminata and standard.
Nitric oxide scavenging activity: Nitric oxide scavenging activity was determined by the earlier method.21. Nitric oxide generated by sodium nitroprusside is converted into nitrous acid in contact with air and it can be easily measured using Greiss reagent. Sodium nitroprusside (5mM) in PBS was mixed with different concentrations of various extracts and incubated at 25°C for 150min. The samples from the above were reacted with Greiss Reagent (1% sulfanilamide, 2% H3PO4, and 0.1% NDD). The absorbance of the chromophore formed during diazotization of nitrite with sulfanilamide and subsequent coupling with NED was read at 546 nm using a UV Spectrophotometer and referred to the absorbance of standard solutions of potassium nitrite treated in the same way with Greiss reagent. The blank consisted of PBS and equal ratio of sodium nitroprusside and Greiss reagent treated in an identical manner without the test sample.
The results of this study are presented in tables 1–3 and Figures 1–9 as mean ±SEM, (standard error of the mean.
DPPH radical scavenging activity
Stem bark extracts of Dhaura using different solvent systems inhibited the generation of DPPH free radicals with increasing concentration up to 120μg/ml for chloroform (77.24%) and aqueous (88.85%) extracts (Table 1) (Figures 1&3). A further increase in the chloroform and aqueous extracts led to a decline in the DPPH scavenging activity (Figures 1&3). The highest scavenging activity of 92.71% was detected for ethanol stem extract of Dhaura at a concentration of 80µg/ml (Table 1) (Figure 2). This concentration was 40μg/ml lower than chloroform and aqueous extracts, respectively (Table 1). The ethanol extract was superior in scavenging the DPPH free radical when compared to chloroform and aqueous extracts (Table 1).
Concentration |
Percent inhibition (mean±SEM) |
||
Anogeissus acuminata Extract type |
|||
Choloroform |
Ethanol |
Aqueous |
|
20 |
33.73±1.32 |
57.34±0.26 |
72.76±0.09 |
40 |
44.11±0.17 |
88.12±0.65 |
76.66±0.11 |
60 |
53.37±0.66 |
92.65±0.13 |
84±0.16 |
80 |
69.18±0.92 |
92.71±0.45 |
87.04±0.25 |
100 |
74.01±0.62 |
88.77±0.41 |
88.38±0.094 |
120 |
77.24±0.66 |
8721±0.11 |
88.85±0.16 |
140 |
67.77±0.36 |
---- |
80.66±0.013 |
160 |
63.14±0.17 |
---- |
79.91±0.19 |
180 |
---- |
---- |
78.95±0.09 |
200 |
---- |
---- |
76.76±0.25 |
Table 1 DPPH radical scavenging activity of chloroform, ethanol and aqueous extracts of Anogeissus acuminata
Superoxide scavenging activity
Various extracts of Dhaura inhibited the production of superoxide anion radical in a concentration dependent manner (Table 2). The maximum scavenging of superoxide radical was recorded for chloroform (76.43%) and aqueous (93.86%) extract at a concentration of 140µg/ml, respectively (Table 2) (Figure 4&6). The ethanol extract (96.34%) also inhibited the generation of superoxide anion radical and a maximum neutralization of superoxide anion radical was observed at 200µg/ml (Table 2) (Figure 5). The chloroform extract was least effective when compared to ethanol and aqueous extracts (Table 2).
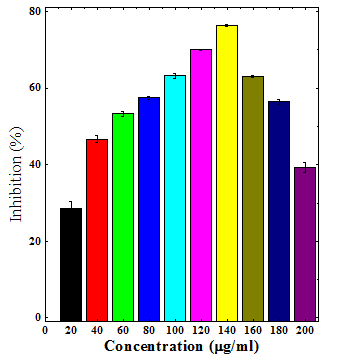
Figure 4 Superoxide radical scavenging activity of chloroform stembark extracts of Anogeissus acuminata.
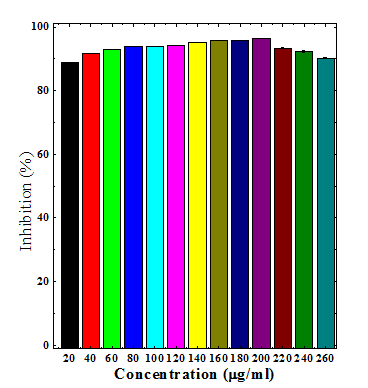
Figure 5 Superoxide radical scavenging activity of ethanol stembark extracts of Anogeissus acuminata.
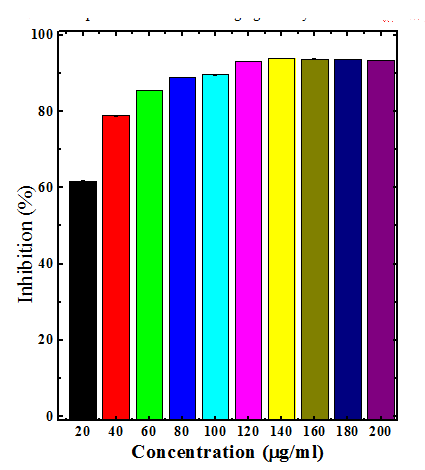
Figure 6 Superoxide radical scavenging activity of aqueous stembark extracts of Anogeissus acuminata.
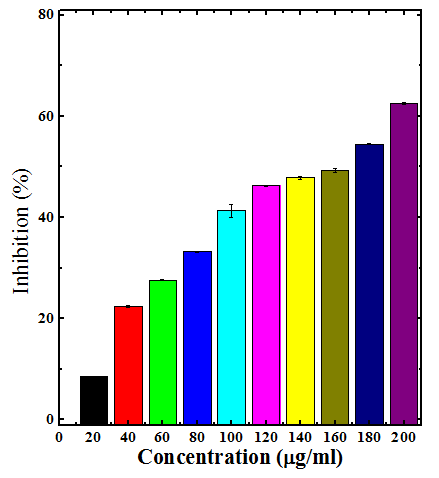
Figure 7 Nitric oxide radical scavenging activity of chloroform stembark extracts of Anogeissus acuminata.
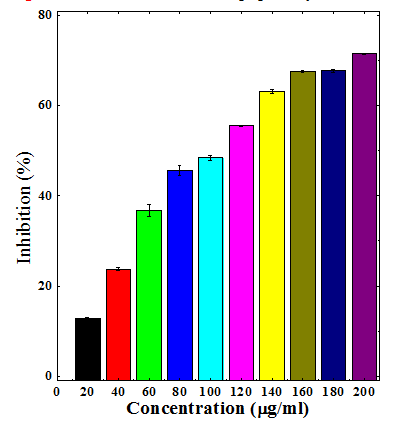
Figure 8 Nitric oxide radical scavenging activity of ethanol stembark extracts of Anogeissus acuminata.
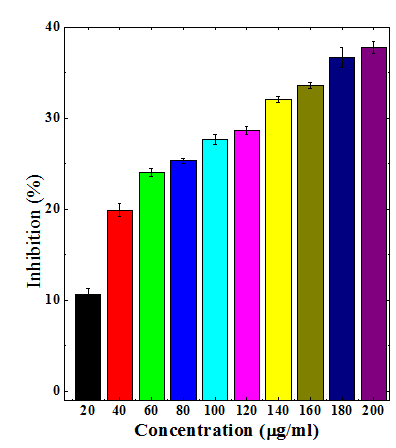
Figure 9 Nitric oxide radical scavenging activity of aqueous stembark extracts of Anogeissus acuminata.
Concentration |
Percent inhibition (mean±SEM) |
||
Anogeissus acuminata Extract type |
|||
Choloroform |
Ethanol |
Aqueous |
|
20 |
28.66±1.72 |
88.84±0.08 |
61.69±0.20 |
40 |
46.69±0.97 |
91.71±0.09 |
78.82±0.13 |
60 |
53.25±0.75 |
92.97±0.04 |
85.54±0.08 |
80 |
57.45±0.31 |
93.77±0.01 |
88.95±0.06 |
100 |
63.21±0.63 |
93.91±0.01 |
89.61±0.09 |
120 |
69.99±0.15 |
94.34±0.02 |
93.02±0.02 |
140 |
76.43±0.34 |
95.05±0.03 |
93.86±0.01 |
160 |
62.98±0.23 |
95.74±0.07 |
93.78±0.01 |
180 |
56.45±0.66 |
95.82±0.01 |
93.55±0.02 |
200 |
39.31±1.32 |
96.34±0.001 |
93.38±0.03 |
220 |
|
93.42±0.13 |
|
240 |
|
92.34±0.18 |
|
260 |
|
90.27±0.07 |
|
Table 2 Superoxide radical scavenging activity of chloroform, ethanol and aqueous extracts of Anogeissus acuminate
Nitric oxide radical scavenging activity
The different extracts of Dhaura led to dose dependent rise in the inhibition of nitric oxide formation (Table 3) (Figures 6–9). The maximum scavenging of nitric oxide radical was observed for chloroform, ethanol and aqueous extracts at a concentration of 200µg/ml (Table 3) (Figures 7–9). The aqueous extract was least effective when compared to ethanol and chloroform extracts (Table 3).
Concentration |
Percent inhibition (mean±SEM) |
||
Anogeissus acuminata Extract type |
|||
Choloroform |
Ethanol |
Aqueous |
|
20 |
8.52±0.09 |
12.87±0.19 |
10.59±0.68 |
40 |
22.37±0.24 |
23.76±0.33 |
19.89±0.69 |
60 |
27.62±0.09 |
36.74±1.37 |
24.03±0.44 |
80 |
33.23±0.12 |
45.65±1.04 |
25.32±0.25 |
100 |
41.29±1.28 |
48.41±0.47 |
27.64±0.51 |
120 |
46.25±0.18 |
55.55±0.11 |
28.68±0.44 |
140 |
47.84±0.33 |
63.14±0.44 |
32.04±0.32 |
160 |
49.25±0.35 |
67.54±0.22 |
33.59±0.33 |
180 |
54.49±0.16 |
67.76±0.29 |
36.69±1.12 |
200 |
62.54±0.18 |
71.51±0.11 |
37.72±0.68 |
Table 3 Nitric oxide radical scavenging activity of chloroform, ethanol and aqueous extracts of Anogeissus acuminate
The aerobic organisms utilize oxygen for production of chemical energy for their daily requirements. The utilization of oxygen during oxidative phosphorylation in mitochondria produce superoxide free radical, which is converted into hydrogen peroxide. Hydrogen is less toxic but is long lived and produces long term toxic effect in the tissues if not removed.22–24 However, free radicals are essential for cell signaling and to fight against the invading bacteria.24,25 Despite the fact that free radicals play an important beneficial role in cellular physiology if these free radicals are not neutralized by the endogenous system, they cause oxidative stress. The oxidative stress is indicated in inflammation and several diseases including autoimmune, cardiovascular and neurodegenerative disorders, aging, cancer rheumatoid arthritis, cataract, and diabetes.26,27 This indicates the requirement of countermeasures, which can reduce the excess production of free radicals by using pharmacological agents that are able to restrain or neutralize the formation of free radicals thereby reducing the frequency of oxidative stress induced diseases. Therefore, the present study was performed to investigate the free radical scavenging activity of stem bark extract of Dhaura, Anogeissus acuminata.
Estimation of DPPH scavenging is a convenient method to study the antioxidant activity of any pharmacological agent. 1,1–diphenyl–2–picrylhydrazyl (DPPH) possesses a free electron and is violet coloured, which changes its colour to yellow when it accepts electron from any antioxidants and is converted into DPPH–H. Stem bark extract of Dhaura using different solvent systems inhibited the generation of DPPH free radicals in a concentration dependent manner. Various extracts of Dhaura showed a concentration dependent elevation in the scavenging of DPPH radical up to 120µg/ml for chloroform and aqueous extracts and 80µg/ml for ethanol extract. Similarly, the chloroform, ethanol and water extracts of Croton caudatus, Colocasia gigantea, Oroxylum indicum, Schima wallichii and Mimosa pudica have been reported to scavenge DPPH radical in a dose dependent fashion.28-31. The other plant extracts Including Aegel marmelos, Syzygium cumini, Milletia pachycarpa, Eleagnus caudata, Dysoxylum gobara, Castanopsis indica and Mimosa pudica have been found to inhibit the generation of DPPH radicals earlier.32–38 This DPPH scavenging activity may be due to the presence of flavonoids and other polyphenols in the Dhaura.15
The superoxide anion free radical is generated as an intermediate during cellular respiration, which is produced as a result of incomplete metabolism of oxygen.39,40 The superoxide anion produces H2O2, which in turn generates hydroxyl free radicals in the presence of metal ions, which are highly reactive and damage important cellular macromolecules like lipids, DNA and proteins.41–43 Thus, neutralization of superoxide radical will inhibit the chain of ROS generation and protect cells from oxidative stress. The chloroform, ethanol and water extracts of Dhaura have been found to inhibit the production of superoxide radical in a concentration dependent manner. The chloroform, ethanol and water extracts of Croton caudatus, Colocasia gigantea, Oroxylum indicum, Schima wallichii and Mimosa pudica have been reported to scavenge superoxide radical in a dose dependent fashion.28–31 The superoxide radical scavenging activity of Dhaura may be due to the presence of several phytochemicals including flavonoid and polyphenols which have been detected earlier.15
The nitric oxide radical is a labile molecule, which is generated in mammalian cells for nerve transmission and to fight infection by neutrophils.22,24,44 However excess production of nitric oxide is toxic, especially after reaction with oxygen or superoxide anion radicals. The reaction products of nitric oxide including NOx and ONOO–(peroxynitrite) are able to inflict severe cellular damage and pathogenesis.45,46 The chloroform, ethanol and aqueous extracts of Dhaura reduced the generation of nitric oxide in a concentration dependent manner. Several plant extracts including Foeniculum vulgare, Citrus limettiodes, Murraya koenigii, Curcuma aromatica Mentha arvensis, Curcuma longa, Gingko biloba, and Zingiber officinale have been reported to scavenge NO radical earlier.34 Certain plant formulations have been also reported to scavenge nitric oxide in a concentration dependent manner.47 Similarly, Alstonia scholaris, Cynodon dactylon, Morinda citrifolia, Tylophora indica, Tectona grandis, Aegle marmelos, Momordica charantia, Phyllanthus niruri, Ocimum sanctum, Tinospora cordifolia, Coleus ambonicus, Vitex negundo, Ipomoea digitate, Boerhaavia diffusa, Picrorrhiza kurroa Santalum album and Eugenia jambolana have been reported to passivate NO radicals earlier.48 The NO inhibitory action of Dhaura may be due to the presence different phytochemicals including flavonoids and other polyphenols.15
The chloroform, ethanol and aqueous extracts of Dhaura, Anogeissus acuminata inhibited the generation of DPPH, superoxide and nitric oxide depending on their concentration. This free radical scavenging ability of Dhaura may be due to the presence of flavonoids and other polyphenols. The medicinal use of Dhaura may be linked to its ability to scavenge various free radicals.
A grant from University Grants Commission, Government of India, and New Delhi to GCJ supported the work carried out in this study.
The authors have no conflicts of interest statement to declare.

©2019 Jagetia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.