International Journal of
eISSN: 2381-1803


Review Article Volume 13 Issue 1
1Minor Forest Produce Processing and Research Center, India
2Department of Microbiology, Career College Bhopal, India
Correspondence: Deepak Dwivedi, Minor Forest Produce Processing and Research Center Barkheda Pathani, Bhopal, Pincode–462021, M.P., India
Received: November 25, 2019 | Published: January 9, 2020
Citation: Dwivedi D, Vats N. Comparative study of herbal compounds curcumin and negundoside on the biofilm producing property of streptococcus mutans isolated from oral infection. Int J Complement Alt Med. 2020;13(1):1-5. DOI: 10.15406/ijcam.2020.13.00484
Biofilm is a community of cells attached to either a biotic or abiotic surface enclosed in a complex exopolymeric substance (EPS). Biofilms allow micro–organisms to trap nutrients and withstand hostile environmental conditions by Quorum sensing (QS). Several serious infections and disease are reported as a result of biofilm. This study aimed to evaluate the efficacy of natural compounds Curcumin and Negundoside on the biofilm producing property of Streptococcus mutans. Samples were collected from patients having oral infection and 30 isolates were identified as S. mutans and screened for biofilm formation by using microtiter plate method. Strongest biofilm producer SM03 was used for minimum inhibitory concentration and minimum biofilm inhibitory concentration. Subsequently this concentration used against each of strong biofilm producer isolates at 492<0.5 optical density (OD). The 30 isolates screened for biofilm formation, 18 isolates showed strong biofilm formation, 09 isolates showed moderate formation and 03 isolates showed poor/non biofilm formation. The MIC of Curcumin for the strongest biofilm producer SM03 was, 0.63±0.02 whereas that of Negundoside was 0.48± 0.02 and minimum biofilm inhibition concentration of Curcumin was 0.0570±0.03 and Negundoside 0.0417±0.03 which was lower than the Curcumin. The MBIC of both compounds significantly inhibited biofilm formation of all the 18 strong biofilm-forming isolates. The results of this study demonstrated significant antibiofilm effect of the natural compound Curcumin and Negundoside which contribute towards the development of database for novel drug candidates for treating oral infection caused by S. mutans.
Keywords: mutans, biofilm, natrual compound, curcumin, negundoside
EPS, exopolymeric substance; QS, quorum sensing; AI, autoinducers; BHI, brain heart infusion; MSA, mutant sanguis agar
Biofilm is a community of cells attached to either a biotic or abiotic surface enclosed in a complex exopolymeric substance (EPS).1 Biofilms allow micro–organisms to trap nutrients and withstand hostile environmental conditions by Quorum sensing (QS). It is widespread and well–known cell–to–cell communication phenomenon for the regulation of behaviors of biofilm formation and virulence.2–4 QS Comprises of chemical communication among bacteria involving formation, secretion, detection and reaction to molecules known as autoinducers (AI). Several serious infections are reported to be a result of biofilm formation and further it leads to chronic diseases. These Persistent infections is a challenge for public health on a global scale as it reduces the effectiveness of treatments and increases morbidity, mortality, and health care costs.5 Streptococcus mutans is an impotent pathogen and is a common cause of oral infections such as dental caries; S. mutans effectively utilizes dietary sucrose to synthesize large amounts of exo polysaccharides, which plays an important role in accumulation, adhesion, plaque matrix formation of micro-organism, These processes in most cases lead to serious infections. The ability of micro-organism to from biofilm on host tissue surface is an important step in the development of infection.6 Due to poor hygienic condition and infection of pathogenic micro–organism creates difficulties and presently wide range of antibiotics are being used for treating infections but due to their adverse effect and antibiotic resistance is now paying attention towards natural biologically active herbal compounds as an alternative medicine.7,8 Natural compounds Curcumin commonly found in Curcuma longa it’s a diarylheptanoid, the principal component of Curcuma longa. Negundoside it’s a type of glycoside found in Vitex negundo are available plant species in India. It is a reputed medicinal herb and employed as a traditional cure in Asian system of medicine (Indian, Chinese and Malaysian) for variety of disease conditions.9–12 The objective of present study was to identify natural compounds like Curcumin and Negundoside for their potential against biofilm producing activity.
Bacterial Isolation and Identification
Streptococcus mutans were isolated from dental caries or plaque from patients of OPD’s Peoples Dental Academy, Bhopal, M.P., India. These patients were of both sexes with the mean age of 20 years. The standard strain was S. mutans ATCC 25175. All samples were cultured on the media such as Brain Heart Infusion agar (BHI, Himedia Laboratories, India) in a 5% CO2 enriched atmosphere and Mutant Sanguis Agar (MSA, Himedia Laboratories, India). The biochemical tests were done for their identification. Among 20 samples from patients having dental caries, 30 isolates were identified as S. mutans.
Preparation of natural compounds
Natural compounds Curcumin and Negundoside were procured from Natural remedies India, were dissolved at a concentration 10mg/ml in dimethylsulfoxide (DMSO, Mark).
Screening of S. mutans for biofilm formation
Microtiter plate method- Quantification of S. mutans isolates biofilm formation was carried out by using microtiter plate method. To assay biofilm formation of the S. mutans isolates, an overnight culture of each was grown in brain heart infusion broth (Himedia Laboratories, India) for 18–20 h at 37°C. One ml of each overnight culture was transferred to 10ml of sterile BHI broth with the addition of 1% Sucrose for biofilm production. The suspensions were adjusted with the same BHI medium to 0.5 on the McFarland turbidity standard as measured by absorbance (0.08–0.1 at 625nm) in a spectrophotometer (Shimadzu, Australia), corresponding to approximately 102 cfu/ml. Then, from each culture, 250μl volumes were transferred into wells of a microtiter plate (Himedia Laboratories, India).13 Blank wells contained broth, only. Plates were made in triplicate and incubated for 24h at 37°C. At the end of 24h, the planktonic suspension and nutrient solution were aspirated and each well was washed three times with 300μl of sterile physiological saline. The plates were strongly shaken in order to remove all non–adherent bacteria. The remaining attached bacteria were fixed with 250μl of 96% ethanol per well and, after 15 min, plates were made empty and left to dry. Each well was then stained for 5 min with 200 μl of 2% crystal violet (CV Gram stain, Merck, Germany). The stain was rinsed off by placing the plates under running tap water. After drying the stained plates, biofilms were visible as purple rings formed on the sides of each well. The quantitative analysis of biofilm formation was performed by adding 200μl of 33% (v/v) glacial acetic acid (Merck, Germany) per well. Then the optical density (OD) of the stain was measured at 492 nm by an ELISA reader (Lisa, Germany) as described previously.13 Biofilm formation was scored as follows: –, non–biofilm–forming (A492 ≤1); +, weak (1 ≤ A492 ≤ 2); ++, moderate (2 < A492 ≤3); +++, strong (A492 >3). Microtiter assay was performed in triplicate.
Microscopic analysis by using the coverslip method
Biofilm of S. mutans clinical isolates were grown as follows, individual sterile culture dishes were filled with 2.5 ml of BHI broth with 1% sucrose and sterile 18mm diameter glass microscope cover slip was added to each dish, and culture dishes were covered. Each sample was inoculated with defined volume of overnight culture. The dishes were incubated micro aerobically at 37ºC for 48hr. Glass cover slips containing attached biofilm were removed from dishes and rinsed briefly with PBS and stained with 0.5% crystal violet for 5min. Stained biofilm were observed microscopically.14
Determination of minimum inhibitory concentration of curcumin and negundoside
The minimum inhibitory concentration (MIC) of natural compounds Curcumin and Negundoside were evaluated on all the isolates by broth dilution methods. The natural compounds were dissolved in dimethyl sulfoxide (DMSO), initial concentration was 2mg/ml to 0.0078mg/ml. The initial test concentration was serially diluted two–fold. Each well was inoculated with 5μL of suspension containing 108cfu/mL of bacteria. The plates with bacteria were incubated for 24h at 37oC. After incubation, 5μL of tested broth was placed on the sterile BHI plates and incubated at respective temperature. The MIC for bacteria was determined as the lowest concentration of the extracts inhibiting the visual growth of the test cultures on the agar plate. Triplicates were maintained.15,23
Biofilm inhibition assay in presence of curcumin and negundoside
Only those isolates of S. mutans that were classified as strong biofilm producers were used in the biofilm inhibition assay. Test compounds were dissolved in DMSO (10mg/ml), and two fold dilutions were made to result in a final concentration ranging from 2–0.0078 mg/ml in the wells after the addition of the freshly diluted brain heart infusion broth culture containing 106cfu of the strong biofilm forming isolates per well. After incubation for 24h at 37ºC, microtiter plate was washed, fixed and biofilms were stained and visualized as outlined above. The inhibitory effect of the plant compound on biofilm production was calculated by subtracting the media control. The biofilm inhibition concentration (BIC) is the concentration of the natural compound at which the biofilm formation was reduced to an absorbance 492<0.5 OD. Each assay for BIC determination was performed in triplicate.
Statistical analysis
Calculations and statistics were performed using GraphPad 5.0 software (GraphPad Software Inc., La Jolla, CA). The results were analyzed using one–way analysis of variance (ANOVA). Significance was defined as P < 0.05. Results are presented as mean ± the standard error of the mean (S.E.M.).
Screening of S. mutans for biofilm formation
The 30 S. mutans isolates were screened for biofilm formation by the microtiter plate method and the results are shown in Figure 1, all 30 isolates were classified by their biofilm forming potential as follows: 18 isolates were strong biofilm producers, 9 isolates were shown moderate producer and 3 isolates were shown poor/non biofilm producers. The S. mutans ATTCC was included as an assay control and found it to be a moderate biofilm producer. The observations confirm that the magnitude and intensity of biofilm formation of 18 isolates were significantly greater than those of the poor/nonbiofilm producer. The result of Figure 1 also shows the quantitative evaluation of identifying and demarcating strong biofilm producing S. mutans isolate from moderate and poor/nonbiofilm producing isolates. The biofilm forming potential of the strong producers at OD492 was greater than 1, whereas non–biofilm producing isolates at OD492 was less than 1. The isolate SM03 was identified as the strongest biofilm producer, whereas SM15 and SM27 were identified as moderate and poor/non biofilm producers respectively.
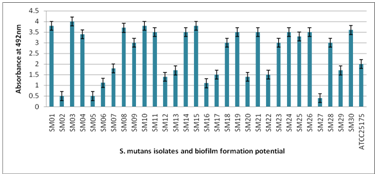
Figure 1 Biofilm formation of 30 isolates of S. mutans by using microtiter plate assay. Poor/non biofilm forming (A492≤1); weak (1≤ A492 ≤2); moderate (2< A492 ≤3); strong (A492 >3).
Microscopic analysis of biofilm formation
For visualization of biofilm formation by three categories of S. mutans isolates (Strong, Moderate, and poor/non biofilm producer), the microscopic slide assay was performed and the results are shown in figure 2. The biofilm formation was clearly visible for the strong biofilm producer SM03 followed by the moderate biofilm producer SM15 at 48 h and the poor/non biofilm producer SM27 did not form the biofilm even after 48 h. The strong biofilm producer also showed strong adherence to the slide and thus one of the strongest biofilm producer SM03 isolate was selected for evaluating the MIC and BIC.
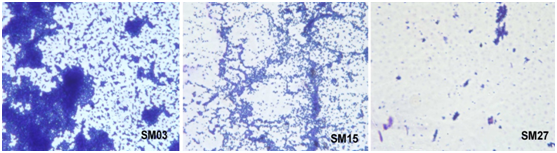
Figure 2 Microscopic visualization of Biofilm Production, where SM03 - Strong Biofilm Producer, SM15 - Moderate and SM27 - Poor/non Biofilm Producer.
Determination of MIC and BIC of curcumin and negundoside
The results of Minimum Inhibitory Concentration (MIC) and Minimum Biofilm Inhibitory Concentration (MBIC) of the natural compounds analyzed for the strongest biofilm producer isolates SM03 are presented in Table 1. A significance difference in the MIC and BIC of the natural compounds was noted: the MIC of Curcumin are 0.63±0.02 whereas that of Negundoside was 0.48±0.02 (MIC Negundoside<Curcumin). The Minimum Biofilm Inhibitory Concentration (MBIC) of Curcumin was 0.0570±0.03, whereas that of Negundoside was 0.0417±0.03. These above results confirm that the Negundoside is the most potent antimicrobial and antibiofilm compound (Negundoside>Curcumin).
S. No. |
Compound |
Minimum inhibitory concentration (MIC) mg/mL |
Biofilm inhibition concentration (BIC) mg/mL |
1 |
Curcumin |
0.63±0.02 |
0.0570±0.03 |
2 |
Negundoside |
0.48±0.02 |
0.0417±0.03 |
Table 1 The Comparative effect of two different natural compounds on growth inhibition versus biofilm inhibition. The values were determined for the high biofilm forming isolate SM03. Values are mean±S.E.M. of three replicates
Comparative study of herbal compounds Curcumin and Negundoside on the biofilm producing property of Streptococcus mutans isolated from oral infection
Biofilm inhibition of strong biofilm producers
The results of Biofilm Inhibition by the natural compounds Curcumin and Negundoside in each strong biofilm forming S. mutans isolates are presented in Table 2. These results clearly confirms that the MBIC of Curcumin and Negundoside has reproducible biofilm inhibitory activity against each of strong biofilm producing isolates of S. mutans. The comparative effect of Minimum Biofilm Inhibition Concentration of Curcumin 0.0570± 0.03 and Negundoside 0.0417±0.03 at OD492 < 0.5 shows the significant inhibition of biofilm. Therefore data of minimum biofilm inhibition concentration truly reflects Negundoside > Curcumin compounds have potent ability to inhibit biofilm formation.
Isolate No. |
Absorbance at 492nm for Biofilm Inhibition in the presence of natural compounds |
|
Curcumin |
Negundoside |
|
SM01 |
0.17±02 |
0.13±02 |
SM03 |
0.27±02 |
0.22±02 |
SM04 |
0.20±02 |
0.18±02 |
SM08 |
0.23±02 |
0.20±02 |
SM09 |
0.20±02 |
0.23±02 |
SM10 |
0.24±02 |
0.17±02 |
SM11 |
0.19±02 |
0.13±02 |
SM14 |
0.21±02 |
0.11±02 |
SM15 |
0.23±02 |
0.17±02 |
SM18 |
0.20±02 |
0.12±02 |
SM19 |
0.19±02 |
0.21±02 |
SM21 |
0.22±02 |
0.15±02 |
SM23 |
0.21±02 |
0.12±02 |
SM24 |
0.18±02 |
0.13±02 |
SM25 |
0.20±02 |
0.18±02 |
SM26 |
0.17±02 |
0.11±02 |
SM28 |
0.22±02 |
0.14±02 |
SM30 |
0.21±02 |
0.16±02 |
Control ATTCC 25175 |
0.22±02 |
0.23±02 |
Table 2 Biofilm inhibition by the Curcumin and Negundoside in high biofilm forming isolates of S. mutans. Values are mean±S.E.M. of three replicates. (Statistically significant at P<0.05)
The objective of this study was to evaluate natural compounds biofilm inhibition of clinical isolates of S. mutans. Towards this, 30 clinical isolates were screened, out of which 18 isolates strong biofilm producers were identified (Figure 1) the effects of Curcumin and Negundoside were tested for their inhibitory effects on bacterial growth and effect on the biofilm formation in a representative strongest biofilm forming isolate SM03 (Table 1) to determine the minimal concentration required for biofilm inhibition. Further this concentration was used against each of the high biofilm producer isolates.
The comparative effects of Curcumin and Negundoside compounds on growth inhibition versus biofilm inhibition are summarized in Table 1. The biofilm inhibition concentration is significantly lower than the concentration required for inhibition of bacterial growth. Therefore data of minimum biofilm inhibition concentration truly reflect Negundoside > Curcumin compounds have potent ability to inhibit biofilm formation. There are reason behind this, compound tested may have inhibited receptors and molecules involved in the quorum sensing pathway which is required for biofilm formation.20–23
Effects of Curcumin and Negundoside compounds on biofilm inhibition at minimum concentration, data suggested that MBIC value also shows the effective potential to inhibit the biofilm formation by the strong biofilm producer isolates. Absorbance at 492nm for Biofilm Inhibition in the presence of natural compounds suggested that significant potential of biofilm inhibition of Negundoside > Curcumin (Table 2).
Comprehensive analysis of the effect of Curcumin and Negundoside compounds on strong biofilm forming isolates of S. mutans. The results show that both the natural compound has potent biofilm inhibition. It would be interesting to find out the mode of action or cell to cell inhibition action to inhibit biofilm formation. These compounds would be very useful in controlling biofilm forming infection of S. mutans.
The authors are grateful to the Department of Microbiology, Barkatullah University, Bhopal M.P. for laboratory support.
Author declares there are no conflicts of interest.
None.

©2020 Dwivedi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.