International Journal of
eISSN: 2381-1803


Research Article Volume 15 Issue 3
1 Department of Chemistry and Biochemistry, St. Joseph University, USA
2 Department of Biological Sciences, St. Joseph University, USA
3 Department of Chemistry and Biochemistry, Rowan University, USA
Correspondence: Bela Peethambaran, Department Biological Sciences, University of Sciences, 600S 43rd street Philadelphia, PA 19104, USA
Received: May 28, 2022 | Published: June 23, 2022
Citation: Leonce CM,Patel A,Gonsai R, et al. Bioassay-guided fractionation and characterization of neuroprotective compounds from the flowers of Achillea millefolium. Int J Complement Alt Med. 2022;15(3):160-168. DOI: 10.15406/ijcam.2022.15.00605
Oxidative stress is known to play a vital role in the progression of neurodegenerative diseases. The current treatment method primarily targets symptoms by using anti-Parkinson drugs such as levodopa, carbidopa, dopamine (DA) agonists, monoamine oxidase type B inhibitors, and anticholinergics to replace DA. However, these drugs pose severe side effects when used for a prolonged time. Hence, there is a need for a concerted effort to develop natural compounds that reduce oxidative stress and help in the management of PD symptoms. This study aimed to investigate the neuroprotective effects of the petroleum ether extract of Achillea millefolium and its fractions on SH-SY5Y and SK-N-SH neuroblastoma cells using Bioactivity-guided fractionation. To isolate the bioactive fraction, the crude extract was separated on gravity column chromatography. Of the twenty-one fractions collected, one of the fractions (Fraction 4) was found to be the most potent neuroprotective to neuronal cells exposed to the neurotoxins, rotenone, and 6-hydroxydopamine. The results showed Fraction 4’s ability to reduce apoptosis by scavenging ROS, maintaining mitochondrial membrane potential, increasing superoxide dismutase activity, and increasing Sirtuin 3 presence and activity. Fraction 4 also showed the ability to reduce cellular apoptosis through the regulation of the AKT cell signaling pathway. The major compounds present in Fraction 4 were analyzed on TOF-LC-MS. Phosphatidic acid (PA 32:0) and four diacylglycerols (DAG) compounds were identified and characterized for their neuroprotective effect.
Keywords: achillea, extract, parkinson’s, neurodegeneration, sirtuin
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disease and usually manifests in patients over the age of 50.1 It is the second most common neurodegenerative disease after Alzheimer’s and currently affects about one million people in the US and about 5 million people worldwide.2 PD affects the dopamine-producing cells of the substantia nigra and causes a range of symptoms including tremors, slurred speech, slow mobility, and loss of posture and balance.3 PD pathology also includes the production of alpha-synuclein-related Lewy bodies which ultimately lead to cell death.4 PD is mainly idiopathic, but a small percentage of cases also have a familial cause which usually includes mutations in specific genes such as Parkin, ɑ-synuclein, Phosphatase, and Tensin Homolog Induced Putative Kinase 1, Parkinsonism Associated Deglycase, and Leucine-Rich Repeat Kinase 2.5 Generally, increased oxidative stress has been linked to the pathology of PD, though its fundamental mechanisms have not been fully elucidated.6,7 Oxidative stress is shown to influence enzyme activity, protein function, mitochondrial membrane potential, and ultimately cellular apoptosis.7,8 Because of this, curtailing oxidative stress in the brain remains an important step in the treatment of PD. However, simply treating patients with antioxidants cannot fully reverse or halt the progression of the disease. Hence, there is a need to develop natural therapeutics that can alleviate oxidative stress and reduce the progression of PD.
PD diagnosis does not usually occur until more than 50% of neurons have already died and there is no known cure.9 PD drugs only treat the symptoms of the disease and associated side effects. Current drugs used in the treatment of PD such as Levodopa is a dopamine precursor, and another drug, Carbidopa, is a Dopamine-decarboxylase inhibitor that prevents the metabolism of dopamine before it reaches the brain. Levodopa and carbidopa also work in combination with catechol-o-methyltransferase inhibitors and monoamine oxidase B inhibitors to prolong the life of dopamine.10 Dopamine agonists, anticholinergics, and deep brain stimulation can also help treat the motor symptoms associated with PD. However, with prolonged use, these drugs produce severe side effects such as dyskinesia and hallucinations.11
The discovery of levodopa has allowed for great strides toward treating PD and the extension of patients’ life. However, because of the side effects of continued use and its inability to cure PD, natural products have been used, individually and in conjunction with drugs, to treat PD and reduce its effects.12 Natural products have been known to decrease tremors and increase mental function. Extracts from Sida cordifolia, Mucuna pruriens, Withania somnifera, and Hyoscyamus niger, have been used to treat PD.13,14 H. niger seed extract, from which the active compounds hyoscine and hyoscyamine were isolated, had an anticholinergic effect. The root extracts of S. cordifolia, M. pruriens, and W. somnifera had antioxidant effects. Other plants such as Ginko biloba, green teas, and berries, have also been used to slow the progression of PD and ease PD symptoms due to their high antioxidant content.
Natural products continue to be essential in the development of new drug compounds. Plants and microorganisms produce highly specific metabolites yet currently, only 6% of over 300,000 plants have been explored pharmacologically.15,16 Our research focuses on the plant Achillea millefolium L. as a possible treatment for PD. A. millefolium is a perennial plant from the Asteraceae family and contains 110-140 sub-species of Achillea that can be found across all continents.17 A. millefolium has been and continues to be used widely to stop bleeding and reduce inflammation. In native American ethnic groups, A. millefolium is used mainly as a dermatological aid for skin wounds and bleeding and as a cold remedy and fever reducer.18
The phytochemistry and general bioactivity of Achillea species and A. millefolium have been extensively studied. Phytochemicals in A. millefolium have been used largely in traditional medicine for various ailments and some of these treatments have been proven scientifically to provide the expected results. This species has been known for its variety of essential oils, such as pinene and bisabolene, and proazulene compounds, specifically chamazulene and achillicin III. A. millefolium also contains various flavonoids, terpenoids, and alkamides. So far, A. millefolium has been shown to have anti-inflammatory, antimicrobial, and anti-ulcerative effects which align with its traditional use as a wound-healing treatment.19,20 One of the mechanisms of the proinflammatory response in our bodies is the production of reactive oxygen species (ROS) at the site of injury.21,22 An anti-inflammatory agent can therefore decrease an inflammatory response by reducing levels of ROS. In a recent study from our laboratory, the petroleum ether extract from A. millefolium flower was found to be anti-bacterial, antioxidant, and anti-inflammatory.23 We hypothesize that A. millefolium can reduce ROS produced in neurodegenerative disease because of its noted anti-inflammatory response.
Chemicals and general experimental procedures
1,2-dipalmitoyl-sn-glycero-3-phosphate monosodium salt (PA 32:0), penicillin/streptomycin, L-ascorbic acid, 6-hydroxydopamine hydrochloride (6-OHDA), Ethylenediaminetetraacetic acid (EDTA), Diethyl ether (Et2O), 2’, 7’-Dichlorodihydrofluorescin diacetate (DCFH-DA), Hydrogen peroxide 30%, rotenone, Water HPLC grade, and ethyl acetate HPLC grade were purchased from Sigma-Aldrich (St. Louis, MO, USA). Petroleum Ether 35/60 (PE) was purchased from Alfa Aesar (Tewksbury, MA, USA). Methanol HPLC grade was purchased from VWR chemicals (Radnor, PA, USA). Heat inactivated fetal bovine serum was purchased from CPS serum (Parkville, MO, USA). Dimethyl sulfoxide (DMSO), bovine serum albumin (BSA), 4′,6-diamidino-2-phenylindole (DAPI) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) dye were purchased from ThermoFisher (Waltham, MA, USA). Phosphate buffered saline (PBS), Dulbecco’s Modified Eagle Medium (DMEM) F12/Ham media, and 0.05% Trypsin-EDTA was purchased from Corning (Corning, NY, USA). Hydrochloric acid was purchased from Pharmco-Aaper (Brookfield, CT, USA). Silica Gel mesh 230x400 was purchased from Sorbtech (Norcross, GA, USA).
Plant materials and sample preparation
A. millefolium flowers were weighed, frozen with liquid nitrogen then crushed to a powder in a mortar and pestle. PE in a 1:10 ratio of plant mass (grams) to ether volume (ml) was added and refrigerated for 24 hours. The supernatant was collected and refrigerated separately. The extraction was repeated two more times for a total of three extractions. All the supernatants were pooled together, filtered, and the solution dried on a Büchi Rotavapor R-200 (New Castle, DE, USA) with a water bath temperature of 25°C and used for chromatography separation. The plant mass was then dried in an oven at 37 °C and then extracted with ethyl acetate, methanol, and water serially for a thorough extraction of all plant compounds. Dried crude extracts were stored at -20ºC until needed.
The PE crude extract was run on a gravity column to separate the crude extract into smaller fractions. Silica gel was used for the column packing with a mixture of PE-Et2O (1:1) used for the slurry and mobile phase. After packing the column, the PE was introduced to the column, and fractions were collected in 10 ml volumes in test tubes. After 10 samples were collected, the eluting solvent was changed from the PE-Et2O (1:1) to ethyl acetate to elute the more polar compounds. Twenty-one samples were collected and evaluated for cytotoxicity using MTT assays. F4 was the sub-fraction collected at the 4th minute. All fractions were vacuum dried, and their dry weight was used to calculate concentrations.
Cell culture
The human SH-SY5Y and SK-N-SH neuroblastoma cell line was obtained from ATCC (Catalog# CRL-2266, HTB-11). Cells were grown in DMEM F12 supplemented with penicillin/streptomycin, 1 % final concentration (100 IU/ml, 100 μg/ml, respectively), and 10% heat-inactivated fetal bovine serum, and the medium was changed every two days. Cells were maintained at 37°C in a CO2 incubator with a saturated humidity atmosphere containing 95% air and 5% CO2.
Neuroprotection Assays
Stock solutions of PE crude extract and F4 were prepared in DMSO stored at 4ºC and diluted in media to 1000 μg/ml before each treatment with the final DMSO concentration being less than 0.6 % in the cell culture. Stock solutions of 50 mM rotenone were prepared in DMSO and diluted to 500 μM when needed. Stock solutions of 10 mM 6-OHDA in 0.2 % ascorbic acid were made and diluted to 50 μM in media before treatment. Cells were treated with F4 followed by rotenone (500 μM) or 6-OHDA (50 μM) treatments for 24 h each before assays were performed unless otherwise mentioned. Stock solutions of PA 32:0 standard in FBS (2 mM) were prepared fresh and diluted in unsupplemented media before each treatment. Cells were treated with PA 32:0 for 45 mins followed by 6-OHDA treatments for 24 h before assays were performed.
Evaluating compounds' effect on cytotoxicity
SH-SY5Y were suspended in media and seeded in a 96-well plate at 40,000 cells/well and incubated for 24 h. Crude extracts were dissolved in media to a final concentration of 1000 μg/ml in 6 % DMSO. The extract was serially diluted from 1000-31.25 μg/ml and incubated for 24 h followed by treatment with rotenone (500 μM) or 6-OHDA (50 μM) for 24 h. Cells in media only or 0.6% DMSO were used as negative controls while the toxin-only treated group was used as a positive control. After treatment, cytotoxicity was evaluated using the short MTT assay protocol from Vybrant and the absorbance of the plate was obtained at 540 nm. The crude extracts that were not cytotoxic were separated on gravity column chromatography and the sub-fraction, F4 was tested for cytotoxicity using the same MTT assay protocol.
Evaluating compounds' effect on mediating ROS
The OxiSelect Intracellular ROS Assay Kit (Cell Biolabs, San Diego, CA, USA) was used to determine the compounds’ ability to scavenge ROS. Briefly, SH-SY5Y were seeded in a 96 well plate at 40,000 cells/well. DCFH-DA dye was added according to manufacturer protocol followed by treatment with F4 extract for 1 hour. Specified treatment groups were then treated with 250 µM hydrogen peroxide and fluorescence read every 15 mins for 1 hr and compared to positive and negative controls.
Effects of F4 on Sirtuin-3 (SIRT3) Immunofluorescence Assay
The presence of SIRT3 protein was evaluated using Immunofluorescence. After treatments, cells were fixed with -20℃ methanol for 5 mins, blocked with BSA blocking buffer for 30 mins, and then incubated with primary anti-Sirtuin 3 rabbit antibody (Abclonal, A7307) (Woburn, MA, USA) at 37℃ for 45 mins. The cells were then rinsed 3 times with PBS and incubated with Alexa Fluor 647-conjugated Goat Anti-rabbit IgG (Invitrogen, A-21244) (ThermoFisher Scientific, Waltham, MA, USA) for 45 mins at 37℃ and rinsed three more times with PBS. Cells were then incubated with DAPI at room temperature for nuclear staining and refrigerated for imaging in PBS.
Effects of F4 on SIRT3 Activity Assay
SIRT3 activity levels were assessed using the Abcam SIRT3 Fluorometric Assay Kit (ab156067) (Waltham, MA, USA) using the method for Inhibitor/Activator screening protocol. Fluorescence was measured at 360/460 every 2 mins for 30 mins. F4 (2083 μg/ml) and 6-OHDA (5 μM) were evaluated as test compounds and compared to controls.
Western blot method
To determine the effects of F4 on proteins downstream of AKT, the SH-SY5Y cells were treated with 125 µg/ml F4 and 100 µM 6-OHDA, washed with ice-cold 1x PBS, and lysed with RIPA buffer. Protein concentrations were determined by Bradford Assay using Pierce 660 nm Protein Assay reagent (ThermoFisher, Waltham, MA, USA). Following SDS-PAGE, proteins were then transferred to nitrocellulose membranes, blocked for 1-hour in 5% milk at room temperature then incubated overnight at 4°C with different antibodies. Membranes were then incubated with horseradish-peroxidase-linked anti-rabbit or anti-mouse IgGs (Cell Signaling, Danvers, MA, USA) for 1-hour. Protein bands were detected by chemiluminescence using SuperSignal West Dura Chemiluminescent substrate and a Bio-Rad chemiDoc imaging system. Antibodies: GAPDH (Cell signal, #D16H11), p-GSK3β-ser9 (Cell signal, #D17D2), GSK3β (Cell signal, #D7SD3) p-AKT-ser473 (Cell signal, #9271S), AKT (Cell signal, #C67E7), Bcl-2 (Cell signal, #D17C4). Densitometry was performed using ImageJ.
Liquid chromatography-MS (TOF-LC-MS)
F4 and PA 32:0 standard was dissolved in LC-MS grade methanol and filtered before running on LC-MS. F4 was separated on an Agilent ZORBAX Extended C-18 column (1.8 µm, 2.1 mm X 50 mm) (Santa Clara, CA, USA) at a flow rate of 0.25 ml/min for 10 mins in 100% methanol and a 3 μL sample injection volume. Electrospray ionization-time of flight/MS (ESI-TOF) was performed on the Agilent LC-MS TOF 6230 series equipped with a Dual AJS ESI (Santa Clara, CA, USA). The TOF mass analyzer was operated in both positive and negative ion mode using fast polarity switching. MS-MS fragmentation was achieved at 175V and skimmer cone 65V with a scan range from 100-1700 m/z. Nebulizer gas was maintained at 40.0 psi, and dry gas at 8.0 L/min with the dry gas temperature at 325oC.
Cytotoxicity of the Isolated compounds tested using MTT assay
SH-SY5Y cells were cultured as previously mentioned. After incubation for 24 h, cells were then treated with commercially purchased PA 32:0 at 200-12.5 μM serial dilution for 45 mins. PA 32:0 was prepared by suspending in 100% FBS that was diluted to a final concentration of 200 μM PA 32:0 and 10% FBS in unsupplemented media. Cell viability was then evaluated by incubating in 10 μL of 0.5 mg/ml MTT dye solution in 100 μL of fresh media for 2 hours and absorbance was obtained at 540 nm on a spectrophotometric plate reader. Cell viability was also evaluated after the addition of 6-OHDA (50 μM) for 24 hours after pretreatment with PA 32:0 to determine its neuroprotective ability. The positive control in these experiments was the SH-SY5Y cells treated with only 6-OHDA and the negative control was the cells treated with vehicle DMSO. All experiments included three technical replicates and included an average of three biological replicates.
Neuroprotective effect of PA 32:0 on SH-SY5Y
The OxiSelect Intracellular ROS Assay Kit (Cell Biolabs, San Diego, CA, USA) was used to determine PA 32:0 ability to scavenge the ROS produced by H2O2 (250 µM) using the above-mentioned procedure. Cells were treated with commercially purchased PA 32:0 for 45 mins then treatment with H2O2 for 1 hour. The Bioscience SIRT3 fluorogenic assay was used to evaluate SIRT3 activity levels. The method for SIRT3 inhibitor/activator screening was used. Commercially purchased PA 32:0 was evaluated at 100 µM and compared to 6-OHDA and control treatments.
Statistical analysis
All assays were performed in triplicates. The mean and the standard error of the mean were calculated for each treatment group and the differences between the groups were compared by one-way analysis of variance (ANOVA) and student t-tests when specified using GraphPad Prism Software. A p-value of less than 0.05 was considered statistically significant.
PE crude extract and subfraction, Fraction 4 (F4), protects SH-SY5Y cells from neurotoxin assault
We first examined the cytotoxic effect of PE crude extract. As shown in Figure 1a, PE crude extract had limited cytotoxicity at concentrations below 125 μg/ml with an EC50 of 156.4 μg/ml. To determine if the PE crude extract was neuroprotective, the SH-SY5Y cells were pretreated with extract followed by treatment with rotenone. The crude extract at 62.5μg/ml was able to rescue approximately 21% of cells from oxidative stress-induced death compared to cells treated with only rotenone (Figure 1b). This suggested the presence of neuroprotective compounds in this crude extract.
Following separation on gravity column chromatography, the PE crude subfractions were tested for cytotoxicity and neuroprotective effect. We observed that only F4 (500-62.5 μg/ml) was not cytotoxic to the cells and was able to rescue 28-45% of cells from the toxic effect of rotenone Figure 1c). F4 (125 μg/ml) was able to rescue 20.50±6.944 % of SH-SY5Y cells from the effect of 6-OHDA (Figure 1d). These results reassured that F4 is not cytotoxic to SH-SY5Y cells and is neuroprotective.
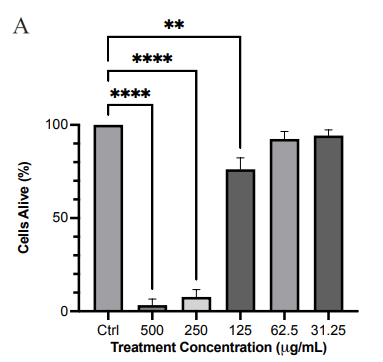
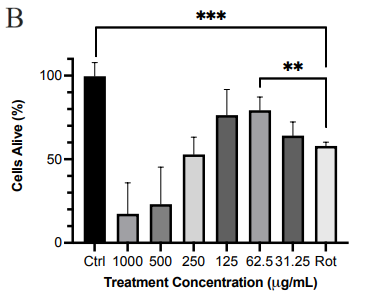
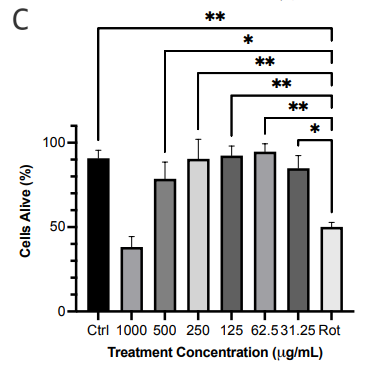
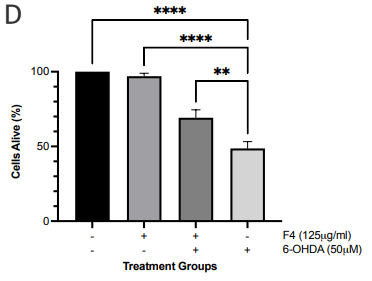
Figure 1 Effect of PE crude extract and F4 on cell proliferation by MTT assay. (a) SH-SY5Y cells were treated for 24 h with various concentrations of PE crude (500-31.25 μg/ml) and cell viability was evaluated. (b) SH-SY5Y cells were treated for 24 h with various concentrations of PE crude (1000-31.25 μg/ml) followed by treatment with rotenone for 24 h. (c) Cell viability was measured after cells were exposed to serially diluted F4 for 24 h followed by treatment with rotenone for 24 h. (d) Cell viability was measured after cells were exposed to 125 μg/ml of F4 and 6-OHDA (50 μM) for 24 h each. **** p < 0.0001 ***p<0.001 **p<0.01 *p<0.05 compared to the control (Fig 1. a, c) rotenone or 6-OHDA only treatment group (Fig 1. b, d).
F4 mediates neuroprotection by scavenging ROS
To determine if F4 is a strong antioxidant, the dye DCFH-DA was employed to investigate levels of ROS after pretreatment of SH-SY5Y cells with the effective dose of F4 (125 μg/ml) before exposure to hydrogen peroxide. DCFH-DA, which is non-fluorescent, is cleaved by various forms of ROS into the fluorescent form, DCF. There is a direct relationship between DCF concentration and levels of ROS in the cells. The controls used in the experiment were SH-SY5Y cells that were treated with hydrogen peroxide, where there was a marked increase in DCF compared to F4 treated cells. The levels of ROS in the F4 treated SH-SY5Y cells were not significantly different from the untreated group. Subsequently, the cells pretreated with F4 followed by treatment with hydrogen peroxide also had a marked difference from cells treated with hydrogen peroxide only, with about a two-fold decrease in DCF concentration of 24.25±7.393 nM (Figure 2a).
F4 increases the presence and activity of mitochondrial SIRT3
SIRT3 levels in the cells were evaluated via immunofluorescence. There was a statistically significant increase in SIRT3 levels in the SH-SY5Y cells pretreated with F4 and the 6-OHDA-only treated group (Figure 2b, 2c). Hence, we also looked at the SIRT3 activity levels. SIRT3 activity was significantly increased in the F4 treatment group compared to the wells that were simulated with oxidative stress using the 6-OHDA (Figure 2d). We observed that the 6-OHDA (50 μM) had an inhibitory effect on SIRT3. However, F4 (2083 μg/ml) was able to increase activity by 17.39±1.935 units.
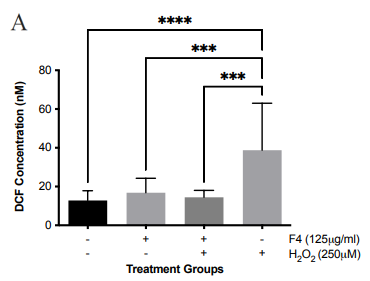
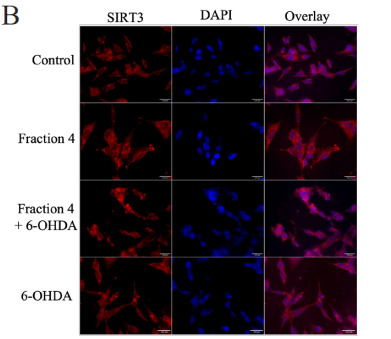
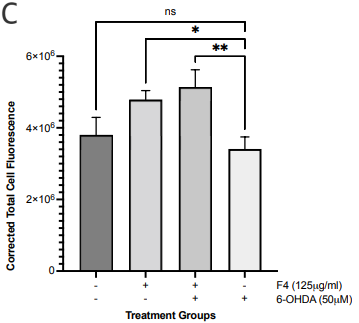
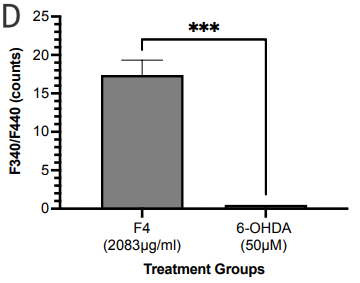
Figure 2 F4 reduces levels of oxidative stress in SH-SY5Y cells. (a) The concentration of DCF produced in SH-SY5Y cells after treatment with F4 and 6-OHDA. DCF levels decreased after pretreatment with F4 in SH-SY5Y cells exposed to 250 μM H2O2. (b) Representative IF images showing a significant increase in SIRT 3 in cells pretreated with F4 compared to cells treated with 6-OHDA only after 24 hours. Nuclei are labeled with DAPI. Scale bar 100 µm. (c) Corrected total cell fluorescence of SIRT 3 images evaluated using Fiji, RRID:SCR_002285 **p<0.01 compared to 6-OHDA only treatment groups. (d) SIRT3 activity was increased after treatment with F4. 6-OHDA did not increase SIRT3 activity and showed an inhibitory effect. The activity was measured using the intensity of fluorescently labeled SIRT3 substrate and net activity compared to control is reported. Negative values were adjusted to zero. **** p < 0.0001 ***p<0.001 **p<0.01 when compared to positive control.
F4 attenuates apoptosis through modulating protein expression in the AKT pathway
To probe the levels of proteins known for their role in pro-survival such as the Wnt pathway 24 western blots were conducted. Neuronal SK-N-SH cells that were pretreated with F4 before 6-OHDA-induced oxidative stress showed an upregulation of p-AKT and BCL-2. Levels of p-AKT, GSK3, and p-GSK3 were reduced in groups treated with 6-OHDA, however, pretreatment with F4 showed a rescue of the protein (Figure 3a-d).
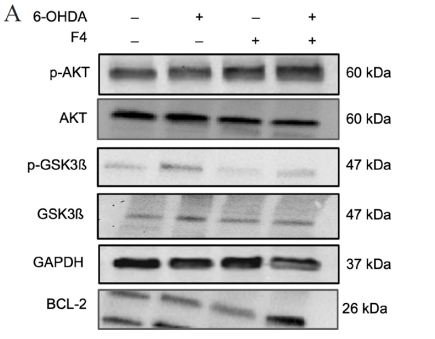
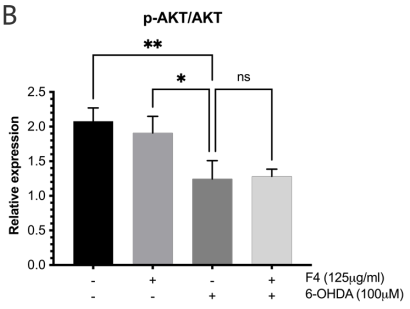
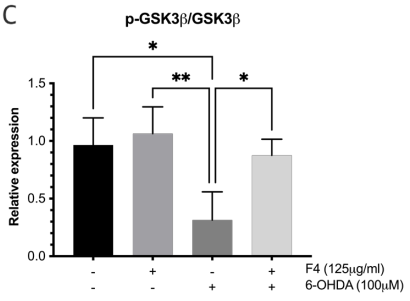
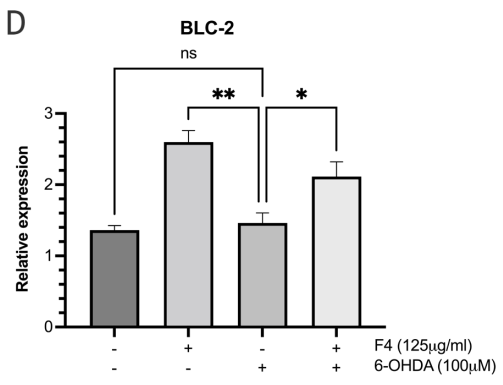
Figure 3 (a) Western blot was used to determine the expression of p-AKT, AKT, p-GSK3B, GSK3B, BCL-2, and GAPDH in SK-N-SH cells. Densitometry graphs of protein bands (b) p-AKT/AKT, (c) p-GSK3B/GSK3B, and (d) BCL-2 normalized to GAPDH.
Separation and identification of compounds from F4 using TOF-LC-MS
F4 was subjected to LC-MS analysis in both positive and negative modes. Six major chromatographic peaks were observed at 0.463, 0.613, 0.829, 1.045, 3.639, and 4.321 mins (Figure 4a) after evaluation in positive mode. The LC-MS of F4 was compared with that of commercially purchased PA 32:0. The LC-MS of this standard sample gave similar chromatographic peaks (Figure 4b). The mass spectrometry (MS) fragmentation patterns for chromatographic peaks at 0.463 and 0.613 mins were obtained (Figure 5,6). It was concluded that one of the major compounds in F4 was PA 32:0 and its neuroprotective ability was verified using bioassays.
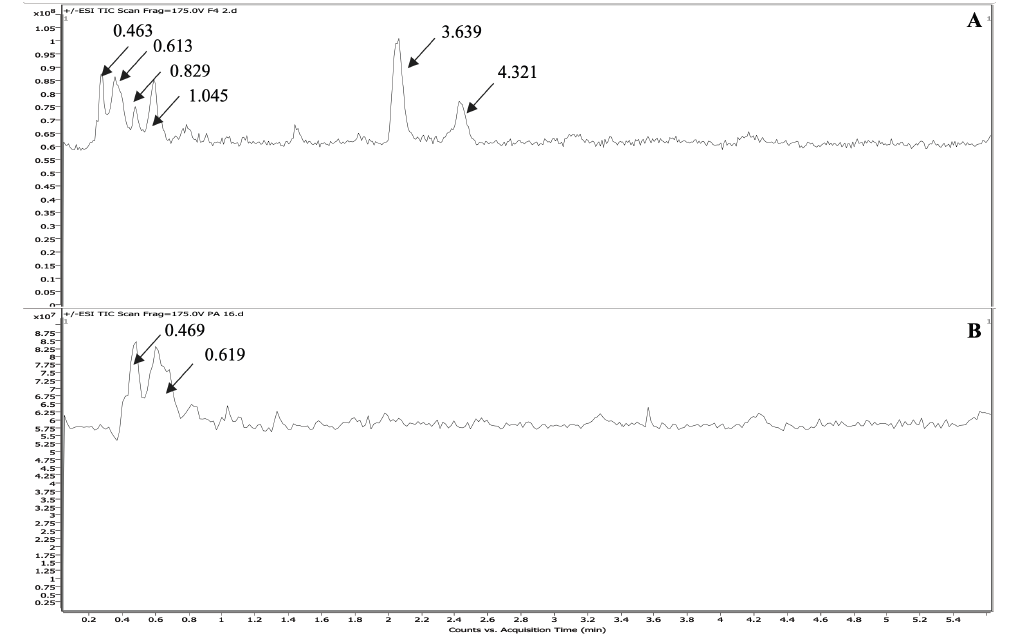
Figure 4 Representative LC-MS chromatograms of (a) F4 and (b) PA 32:0 standard. Key chromatographic peaks are labeled with arrows.
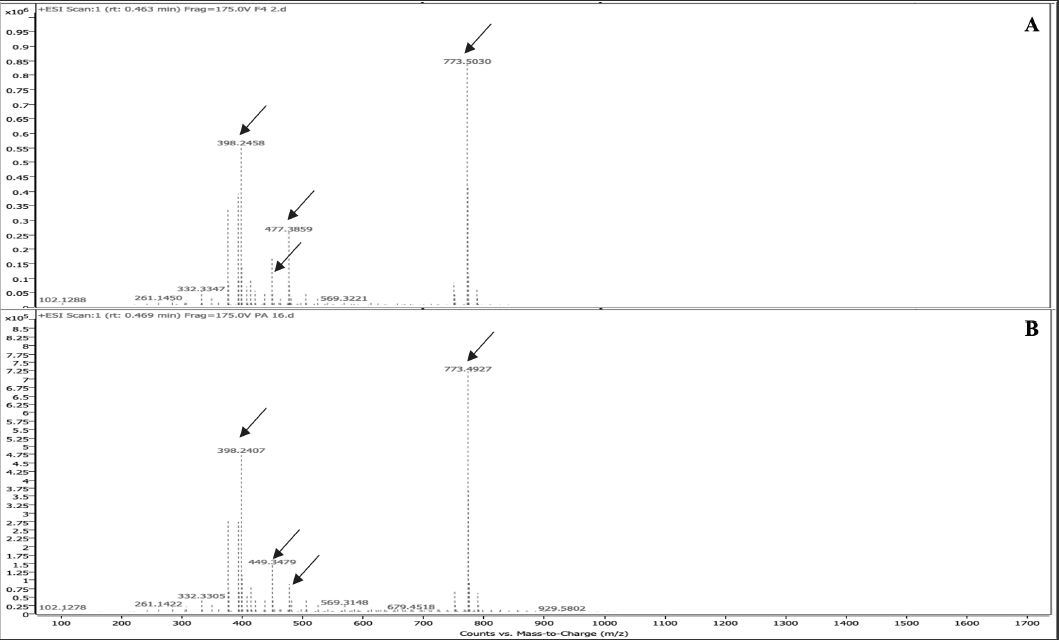
Figure 5 ESI mass spectrum of PA 32:0 and F4 at 0.469 mins. Common peaks at 398.25, 449.35, 477.39, and 773.50 mins are shown with arrows.
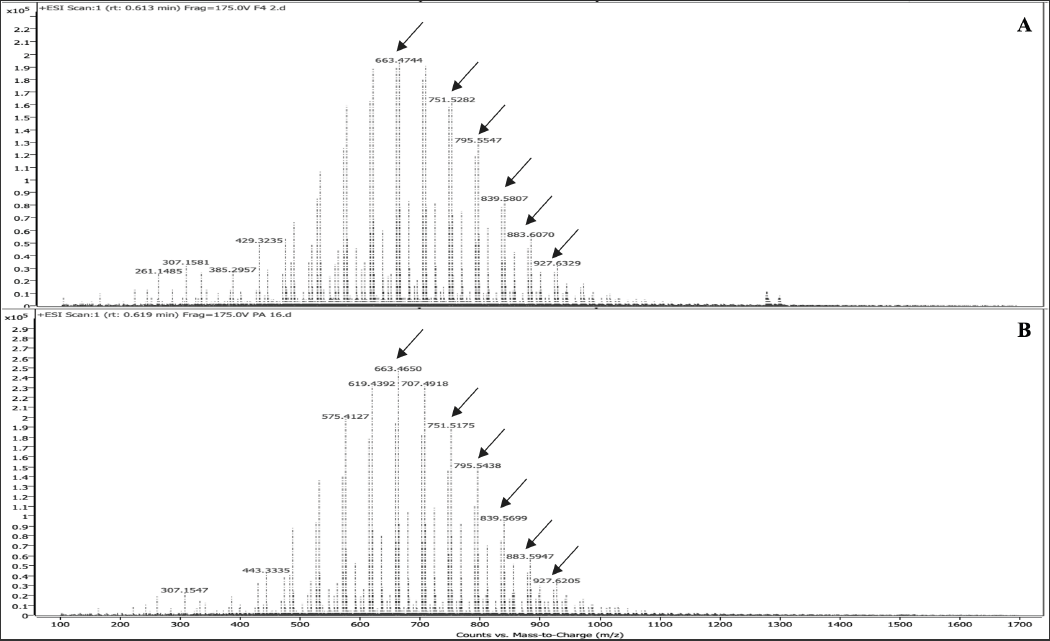
Figure 6 ESI mass spectrum of PA 32:0 and F4 at 0.6 mins. Common peaks at 663.47, 751.52, 795.55, 839.58, 883.61, and 927.62 are shown with arrows.
Using MS, the molecular ion, dimeric ions, and other fragmentation peaks were identified for the peaks at 0.829, 1.045, 3.639, and 4.321 mins in positive mode and identified as various diacylglycerol lipids (DAGs) (Table 1). The fragmentation pattern for Peak No. 3 at 0.892 mins is shown (Figure 7). Structures for the compounds were proposed though the position of unsaturation could not be determined, and the assignment of fatty acid chains to sn-1 and sn-2 positions were suggestive (Figure 8a-d). Figures S1-S21 in the Supplementary Material for spectra in negative mode.
|
m/z (rel. int.) |
|||||||
Peak no. |
RT (mins) |
Mol Spec |
% total area |
M-R2CO+H |
M+H |
M+Na |
2M-R2CO +Na |
2M+Na |
3 |
0.829 |
DG 13:2/7:3 |
6.9 |
284.3348 (19.31) |
391.2888 (25.12) |
413.2720 (100) |
697.4383 (53.22) |
803.5544 (35.58) |
4 |
1.045 |
DG 13:2/8:3 |
13.3 |
284.3341 (8.53) |
419.3207 (38.17) |
441.3031 (100) |
725.4699 (32.48) |
859.6173 (81.82) |
5 |
3.639 |
DG 13:2/22:0 |
36.5 |
284.3350 (4.15) |
|
629.4846 (100) |
913.6504 (11.39) |
1235.9786 (99.29) |
6 |
4.321 |
DG 13:1/22:0 |
11.6 |
|
|
631.5003 (100) |
915.6667 (22.73) |
1240.0108 (23.15) |
Table 1 Chemical composition and fragmentation patterns of F4 DAG compounds using ESI-LC-MS
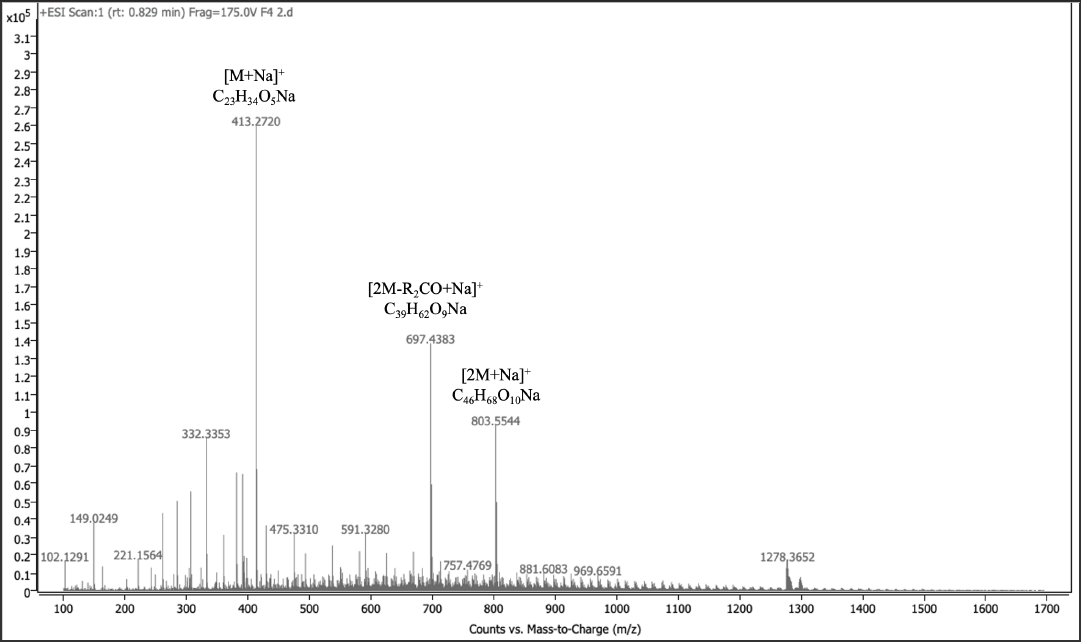
Figure 7 ESITOF-LC-MS positive mode spectrum of F4 DAG in peak No. 3 at 0.829 mins and major fragmentation pathway of protonated DAG 13:2/7:3.
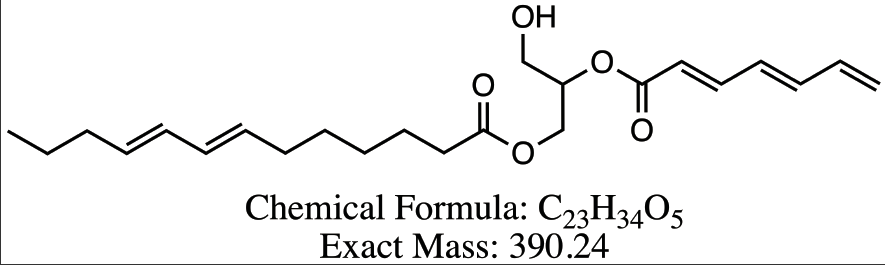
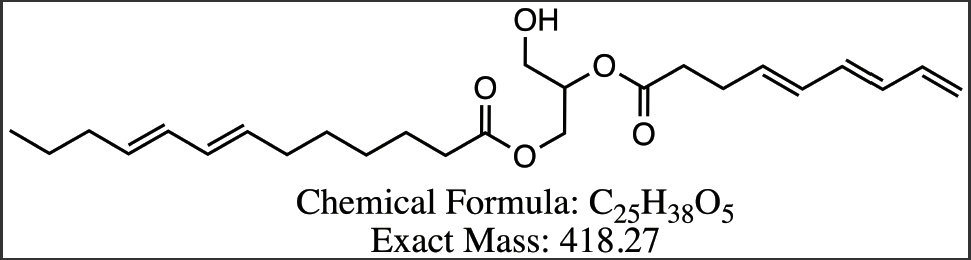
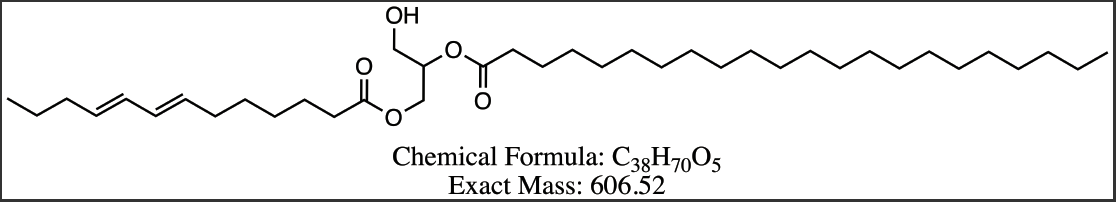
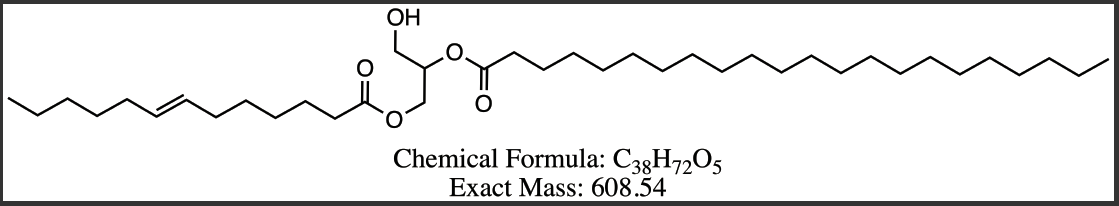
Figure 8 Proposed structures for DAG compounds from Peak 3-6. (a) DG 13:3/7:3 (b) DG 13:2/8:3 (c) DG 13:2/22:0 (d) DG 13:1/22:0.
Phosphatidic acid 32:0 attenuates neurodegeneration by regulating ROS
Cytotoxicity of PA 32:0 was evaluated using the previously mentioned MTT cell viability assay. The assay showed that PA 32:0 was not cytotoxic to the SH-SY5Y cells and there was no significant difference in the cells treated with PA 32:0 or control (Figure 9a). The neuroprotection ability of PA 32:0 was also evaluated against 6-OHDA neurotoxin. We determined that PA 32:0 was able to protect SH-SY5Y cells from the toxicity of 6-OHDA. PA 32:0 was able to reduce 6-OHDA-induced cell death by 22.40±5.032 % (Figure 9b).
PA 32:0 identified as one of the major compounds from F4 was evaluated for its ROS scavenging ability. SH-SY5Y cells treated with PA 32:0 showed limited ROS production when compared to the untreated cell group. Cells pretreated with PA 32:0 were also able to significantly reduce ROS produced in the cells by 21.64±8.231 nM when compared to the H2O2-only treated group (Figure 9c). PA 32:0 was able to increase SIRT3 activity by 19.33±4.764 counts when adjusted from the solvent control and compared to the 6-OHDA treated group (Figure 9d).
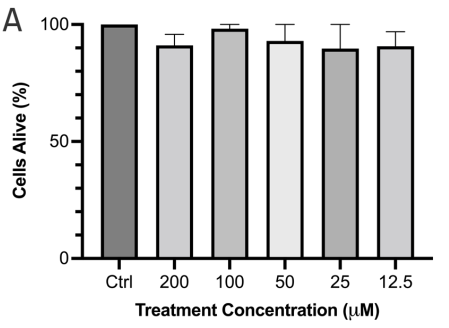
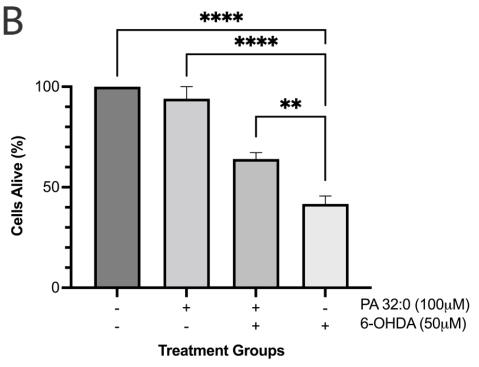
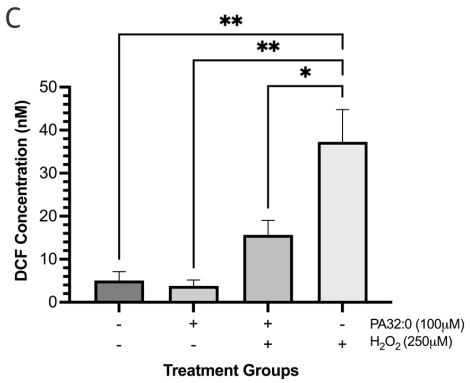
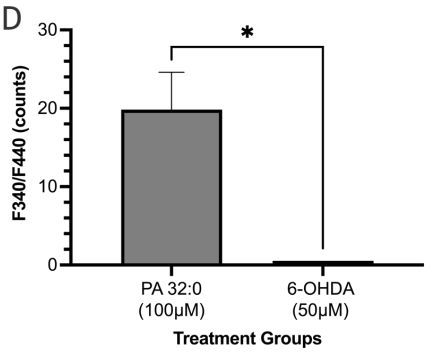
Figure 9 PA 32:0 reduces levels of oxidative stress in SH-SY5Y cells. (a) SH-SY5Y cells were treated for 45 mins with various concentrations of PA 32:0 crude (200-12.5 μM) and cell viability was evaluated. (b) SH-SY5Y cells were treated for 45 mins with PA (100 μM) followed by 6-OHDA (50 μM) treatment for 24 h. (c) The concentration of DCF produced in SH-SY5Y cells after treatment with PA 32:0 and 6-OHDA. DCF levels decreased after pretreatment with PA 32:0 in SH-SY5Y cells exposed to 250 μM H2O2. (d) SIRT3 activity was increased after treatment with PA 32:0. 6-OHDA did not increase SIRT3 activity and showed an inhibitory effect. The activity was measured using the intensity of fluorescently labeled SIRT3 substrate and net activity compared to control is reported. Negative values were adjusted to zero. **** p < 0.0001 **p<0.01 *p<0.05 compared to the 6-OHDA-only treatment group.
In normal physiological conditions, small amounts of reactive oxygen species do not cause damage but coordinate with the body’s antioxidant mechanism to maintain cell homeostasis. However, there is evidence of increased oxidative stress in neurogenerative diseases. Hence, the optimal therapy that can reduce PD symptoms should be a strong antioxidant and could increase the expression of genes in the pro-cell survival pathway. In this investigation, we evaluated compounds extracted from A. millefolium flower extracts to reduce oxidative stress and induce cell survival mechanisms. Rotenone and 6-OHDA in conjunction with MTT assays were used to guide the extraction and isolation of neuroprotective compounds from A. millefolium flowers. A serial extraction was performed from non-polar to polar solvents to extract as many compounds from the flowers as possible. Though not all the extracts were cytotoxic (PE, Methanol, water), only the PE crude extract showed a neuroprotective effect against the SH-SY5Y cells treated with rotenone in a concentration-dependent manner. We have observed the extracts are not cytotoxic but also can protect neurons from simulated oxidative damage.
Neurodegenerative disease pathology, though differing in its modes of progression, have roots in an imbalance or dysregulation of ROS species in the brain and the subsequent oxidative stress that causes such damage.6,25 From the results, we posit that the possible mechanism through which F4 exerted its neuroprotective effect is due to its strong antioxidant effects. We observed that our subfraction was indeed able to reduce the ROS produced by the introduction of hydrogen peroxide to the cells to almost basal levels after treatment with F4 for 1hr.
We also observed an increase in Sirtuin 3 (SIRT3) presence in the cell and an increase in SIRT3 activity when cells were pretreated with F4. SIRT3 is a key regulator protein localized to the mitochondrial matrix and is integral in mitochondrial homeostasis.26,27 SIRT3 has been shown to deacetylase isocitrate dehydrogenase 2 (IDH2), and manganese SOD (MnSOD) to activate both enzymes.28,29 IDH2 plays a role in the production of NADPH, a proton donor used for the regeneration of antioxidants.30 MnSOD is a scavenger of superoxide and works together with catalase to reduce ROS produced from the electron transport chain.31 SIRT3 has also been shown to regulate mitochondrial fatty acid oxidation, regulate mitochondrial Calcium ions, and maintain mitochondrial membrane potential.32–34 Through its activation, SIRT3 can maintain mitochondrial membrane potential which in turn reduces the activation of apoptotic pathways in the mitochondria and ultimately reduces cell death and neurodegeneration.
There is published evidence that the phosphorylated Akt/Akt ratio is significantly reduced in the substantial nigra compacta (SNc) of PD patients.35,36 GSK3 is ubiquitous in the central nervous system but is seen to be abnormally expressed in PD.37 Some studies show that p-AKT inhibits the activity of GSK-3 by phosphorylating ser21of GSK-3α or Ser9 of GSK-3β facilitating survival and growth by inhibiting apoptosis.38 The effect of F4 bioactive fraction on the AKT pathway, which plays a role in regulating the cell cycle showed that cells pretreated with F4 had increased levels of p-AKT and p-GSK3 β which play a role in cell survival.39,40 We also observed F4’s ability to upregulate BCL-2, a protein localized to the outer mitochondrial membrane. BCL-2 inhibits the release of cytochrome C and ROS by the proteins Bax and Bad and limits apoptosis.41–43
We have isolated and identified five lipids from our F4 that show a neuroprotective effect. These include four DAG compounds and PA 32:0 whose neuroprotective activity was evaluated using various biochemical assays. Our results showed that PA 32:0 contributed greatly to the neuroprotective effect of F4 subfraction. PA is ubiquitous in cellular matrixes in low yield and normally conjugated to form other phosphatidyl compounds.44 However, an increase in PA concentration is observed during times of cellular injury.45,46 DAGs also play important roles in the cell and are involved in membrane synthesis, sealing, and signal transduction. They are the main activators of Protein Kinase C (PKC), responsible for the activation/deactivation of enzymes through phosphorylation, and act as membrane sealants through incorporation into the membrane.47,48 This membrane sealing activity could be partially responsible for their neuroprotective activity by maintaining mitochondrial membrane potential and preventing the activation of apoptotic pathways.
PA 32:0 has not been widely researched for its neuroprotective effect when compared to other lipid compounds. The isolated DAG compounds have also not been specifically identified and characterized as secondary metabolites of A. millefolium. PA 32:0 and DAGs remain viable compounds for further exploration as protective compounds for neurodegenerative diseases.
Bioactivity-guided fractionation of our petroleum ether extract from A. millefolium yielded a fraction, F4, that shows neuroprotective ability. F4 can reduce ROS, increase SIRT3 activity, increase SIRT3 presence in the cell and maintain mitochondrial membrane polarization. F4’s neuroprotective ability may arise from its activation of SIRT3, whose role is to deacetylase key enzymes that regulate ROS in mitochondria. PA 32:0 isolated from F4 was able to reduce ROS and attenuate SIRT3 activity which suggests it is a key component of F4 neuroprotection.
The author wishes to acknowledge Dr. Subhash Jonalagadda, Chair, Professor, Rowan University, Chemistry department for giving us access to use the Mass Spectrometry facility.
The authors report no conflict of interest.

©2022 Leonce, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.