International Journal of
eISSN: 2381-1803


Research Article Volume 14 Issue 1
Correspondence: Ayobami O Oyedele, Department of Pharmaceutics, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria
Received: December 02, 2020 | Published: February 16, 2021
Citation: Oyesomi OO, Oyedele AO, Oyemitan IA, et al. Aloe schweinfurthii gel: composition physicochemical and biological properties. Int J Complement Alt Med. 2021;14(1):26-33. DOI: 10.15406/ijcam.2021.14.00529
Aloe schweinfurthii Baker (Asphodelaceae) is an indigenous plant to tropical Africa and cultivated for ethnomedical uses but with scanty scientific support in the literature. This study explored the composition, physicochemical and biological properties of its leaf gel. Phytochemical screening of freshly extracted A. schweinfurthii gel and GC/MS analysis of the freeze-dried gel were performed. Proximate, elemental analyses and physicochemical tests were carried out on the fresh gel in comparison with Aloe vera gel. Acute toxicity screening, analgesic and anti-inflammatory tests were carried out on A. schweinfurthii gel using experimental animal models. The anti-inflammatory activities of the gel, and of A. vera gel as control, were compared. The proximate, mineral element compositions and some physicochemical properties of A. schweinfurthii and A. vera leaf gels gave similar results. Phytochemical tests on A. schweinfurthii gel revealed carbohydrates and flavonoids; while six chemical compounds were identified by its GC/MS analysis, the major one being phytol (44.40%). A. schweinfurthii gel was nontoxic in mice at ≤10,000mg/kg dose levels by oral administration. Doses of 250-750mg/kg in mice demonstrated analgesic activities similar to aspirin at 100mg/kg; while the gel at 1000 mg/kg in both rat and mice models demonstrated anti-inflammatory activities comparable to those of A. vera gel (1000mg/kg) and diclofenac (100mg/kg). The potentials of A. schweinfurthii gel for medicinal applications have thus been validated.
Keywords: Aloe schweinfurthii leaf gel; GC/MS analysis; chemical, proximate, mineral element compositions; physicochemical, analgesic, anti-inflammatory properties.
Aloe schweinfurthii Baker (synonyms: A. barteri Bak.; A. barteri var. lutea A. Chev.; A. trivialis A. Chev.), family Asphodelaceae,1 is the succulent, evergreen, perennial Aloe species indigenous to tropical African countries, which thrives on the soil and in the weather of West African countries including Nigeria.2,3 The plant is cultivated for ethnomedical treatment of intestinal and urinogenital conditions, and applied also externally on sores, wounds and burns. Its edible flowers are sometimes used as a culinary in soups while its sap is added to drinking water for poultry and is said to protect them against avian cholera.3
The genus Aloe has a very long history of medicinal application and exploitation, especially the widely-known species, Aloe vera (L.) Burm. f. (synonym, A. barbadensis Mill.), family Asphodelaceae,1 which is widely cultivated in warm climatic areas of Asia, Europe and America, but originated in tropical Africa.4,5 Different types of extracts from A. vera leaf are used in several plant-based medicinal recipes and products, e.g. its neat gel or leaf extracts (decolorized or not) can be taken orally in the form of a drink meant to promote health or used as an external treatment for burns or other skin injuries; and the extracted, dried latex from the leaf is reported to give laxative or purgative effects.4,5 As a mechanism for its external use in the treatment of burns and other wounds, A. vera gel is thought to increase the rate of healing and reduce the risk of infection.5
On the other hand, a detailed literature search has revealed that little information is known about the possible pharmacological activities of A. schweinfurthii leaf, its gel or extracts, thus making this plant a target for research as well as an unexploited economic and healthcare asset for several developing countries in the West African sub-region. Therefore, the main objective of the present study is to examine the chemical composition, physicochemical characteristics and biological activities namely, antinociceptive and anti-inflammatory activity potentials of A. schweinfurthii leaf gel in laboratory animals.
Plant collection, authentication and extraction
Fresh leaves of Aloe schweinfurthii Baker, family Asphodelaceae, were collected at the medicinal plant garden of the Pharmacognosy Department, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife Nigeria and a voucher specimen, authenticated by the institution’s botanist (Mr. I. I. Ogunlowo), was deposited at the Faculty of Pharmacy Ife Herbarium with the voucher specimen number FPI 2175. The leaf of A. schweinfurthii was cut at the base to separate it from the parent plant and rinsed with potable water; its apex was cut off and the outer rind with the adjacent yellow-juice layer also removed with a sharp knife. The remaining inner-leaf portions (parenchyma) were sliced into smaller pieces (fillets), blended using a Solitaire blender (VTCL Mahavir Impex model, India) and filtered through calico screen to separate the fluid gel from the parenchyma epithelium chaff. The extracted gel in airtight closured amber bottles was kept refrigerated at 4 °C until ready for use within 48 h for subsequent studies.
Aloe vera (L.) Burm. f. (family Asphodelaceae) leaf was collected from the same medicinal plant garden, treated in the same way and its extracted gel used in some of the studies as experimental control sample for subsequent comparison.
Experimental animals
The animals used in this study (both mice and rats) were obtained from the Central Animal House, Department of Pharmacology and Therapeutics, University of Ibadan Nigeria, and from the Faculty of Basic Medical Sciences, Obafemi Awolowo University Ile-Ife Nigeria, after approval of the protocol by the Institutional Animal Ethics Committees. They were maintained and nurtured under standard laboratory conditions of 12/12h night/daylight cycle, temperature (27°±2°C) and relative humidity (70±5 %), fed with standard pellet food, and water ad libitum.
Phytochemical screening of A. schweinfurthii gel
Fresh A. schweinfurthii leaf gel was screened for the presence of carbohydrates by Molisch’s test,6 and for specialized metabolites using different qualitative tests namely: flavonoids (using alkaline reagent tests), tannins (ferric chloride test), sterols (Salkowski test), and saponins by frothing test.7
Gas Chromatography-Mass Spectrometry (GC-MS) analysis of A. schweinfurthii gel
A. schweinfurthii leaf gel (freshly extracted) was freeze-dried in vacuum over 24 h. The freeze-dried gel powder (10.0 mg) was dissolved in methanol (250 μL, AR grade) by a vortex mixer to give a standard solution of the gel (40.0 mg/mL) and used as the test sample for GC-MS analysis.8 The analysis was performed on an Agilent Gas Chromatography (Agilent 7890A, 19091-433 HP), run on a capillary column treated with 5% phenyl methyl siloxane, having calibrated length, internal diameter, and thickness dimensions of 30m, 0.25μm, and 250μm, respectively; and coupled to Agilent Mass Spectrometer (5675C Inert MSD) with Triple Axis Detector. The conditions for analysis were set as follows: column oven temperature was programmed for 35-250°C (temperature at 35°C was held for 5min, raised to 150°C at 4°C/min and then finally to 250°C at 20°C/min and held for 5 min). Helium was used as the carrier gas at a flow rate of 1.5ml/min, with split ratio of 1:100. Other GC-MS conditions were: ion source temperature, 250 °C; interface temperature, 300°C; pressure, 16.2 PSIA; and out time, 1.8 min. A one microliter (1μL) sample volume was injected in split mode, at split ratio of 1:50, and injection temperature of 300°C. The total elution time was 44.5 min. Spectra were obtained in the EI mode with ionization energy of 70eV. The compounds were identified by comparison with the mass spectral standard values obtained from inbuilt NIST library.9
Proximate and elemental analyses of A. schweinfurthii gel compared to A. vera gel
Proximate and mineral elemental analyses of extracted gel from A. schweinfurthii were carried out in comparison to those of A. vera gel. The proximate compositions of the two gels, namely: the crude protein, crude fiber, crude fat, crude carbohydrate (nitrogen-free extract), ash, and moisture contents were determined according to the Association of Official Analytical Chemists (AOAC) methods.10 The crude protein content of each gel was determined by the micro-Kjeldahl method10,11 as described by Pearson,12 while the crude fiber content was determined using the Fibretec hot extraction method.10,13 The AOAC methods used for the moisture and ash contents determination were as described by Nguyen14 while the determination of fat content was carried out using Soxhlet extraction method with petroleum ether (boiling range 40-60°C). The crude carbohydrate was determined by the Difference Method (i.e. 100minus [% Protein + % Fat + % moisture + % ash+% fiber]). All the proximate determinations were carried out in triplicates and the results reported in average percentage values.10 The mineral elements in the gels were determined using Atomic Absorption Spectroscopy (Perkin Elmer Aanalyst 400, Shelton, USA) for iron, magnesium, calcium, manganese, and copper, while Flame Emission Photometry was used for sodium and potassium.15
Physicochemical evaluation of A. schweinfurthii gel compared to A. vera gel
A. schweinfurthii gel and A. vera gel samples were subjected to the following physicochemical tests: density, pH and viscosity determinations. The density of the samples in a 10-mL volumetric flask was determined in triplicate experiments; pH with a digital pH meter (HM Digital Inc, USA), pre-calibrated with standard buffer solutions; while the viscosity of the respective gels was separately determined using the Ostwald-type U-tube viscometer pre-calibrated with the Brookfield standard-viscosity fluid No. 5 procured from Brookfield Engineering Laboratories, Inc. Massachusetts, USA.
Biological activities of A. schweinfurthii leaf gel in animal experiments
Acute toxicity assay of A. schweinfurthii gel in mice: In order to assess the oral acute toxicity profile of A. schweinfurthii leaf gel, its median lethal dose (LD50) was determined following the method described by Lorke16 and Akhila et al.17 as modified: A total of thirteen mice 7-8weeks old weighing between 15 and 20g were used for the study. The doses of the gel required for administration to the respective animals were calculated. The test consisted of two phases. Phase I employed a total of nine (9) mice divided into three (3) groups of three (3) mice each (n=3); while Phase II, which depended on the result of Phase I, employed four (4) mice divided into four (4) groups of n=1.16 The three groups of mice in Phase I were administered oral doses of 10, 100, and 1000 mg/kg body weight (mg/kg b.w.) of A. schweinfurthii gel, respectively, using an oral cannula. The animals were then monitored constantly for the first 6hours, and then intermittently over a period of 24hours in order to record any mortality. The Phase II experiment using four (4) groups of a single animal per group (n=1) was then carried out, following absence of mortality in all the test animals of Phase 1. The Phase II animals were administered oral doses of the gel at 1600, 2900, 5000, and 10,000mg/kg body weight (mg/kg b.w.), respectively.
Evaluation of analgesic potential of A. schweinfurthii gel
Acetic acid-induced writhing in mice: The acetic acid-induced writhing test procedure described by Koster et al.18 and Broadbear et al.19 was used to evaluate the antinociceptive potential of freshly extracted A. schweinfurthii leaf gel. Swiss mice randomly divided into 7 groups (six animals per group: 3male, 3 female; each 7-8 weeks old, weighing 14–22g) were injected through intraperitoneal (i.p.) route with 10mL/kg of 0.3 % (v/v) acetic acid. Sixty minutes before the injection, each mouse was administered orally with a dose (20μL) of A. schweinfurthii gel or control. Group 1 served as the negative control and received 10mL/kg of distilled water, while groups 2, 3, 4, 5, and 6 were pretreated with 100, 250, 500, 750, and 1000mg/kg of Aloe schweinfurthii gel, respectively; and group 7 received diclofenac (75mg/kg) as positive control. The total number of writhing responses during a subsequent 10min period after the administration of acetic acid was recorded for each animal. The percentage inhibition of writhing was calculated by the following formula, using the mean number of writhing per animal group:
Inhibition (%) = ((Number of writhing in control – Number of writhing in test))/(Number of writhing in control ) ×100
Formalin-induced hind paw licking in mice
The procedure was essentially similar to that described by Andrade et al.20 following that of Hunskaar & Hole21 for evaluation of antinociceptive potential of freshly extracted A. schweinfurthii leaf gel. Five groups of Swiss mice (6 animals per group: 3 male, 3 female; each 5-7weeks old, weighing 10–20g) were used. Each mouse was placed in the observation chamber for 5 min before treatment in order to allow acclimatization to the new environment. The mice in Group 1 served as negative control and received 10mL/kg of distilled water i.p.;20 while groups 2, 3, and 4 were orally administered with 250, 500, and 750mg/kg of the Aloe gel, respectively; and Group 5 received acetyl salicylic acid (ASA or aspirin) at 100mg/kg, i.p. as the positive control.20
Following one hour pretreatment with the gel or control sample, 20μL of 1% phosphate buffered formalin was injected subcutaneously under the surface of the right hind paw of each mouse. The duration (in seconds) of the animal’s paw licking, following the formalin administration, was then recorded as an index of nociception, at both the first phase (within 0–5min) indicative of antinociceptive activity, and the second phase (within 15–30 min) indicative of anti-inflammatory activity.21 The mean duration of paw lick behaviour for the six animals in each group of the replicated studies was calculated.
Evaluation of anti-inflammatory activity of A. schweinfurthii gel
Egg albumin-induced rat paw oedema
Anti-inflammatory activity of freshly extracted and 2-day refrigerator-stored A. schweinfurthii leaf gel samples was evaluated using the egg albumin-induced paw oedema test procedure in rat described by Zhao et al.22 Inflammation was induced by the injection of egg albumin (0.1mL) into the sub-plantar tissue of the animal’s right hind paw. The test or control samples were administered to 24h fasted rats 1h before the induction of inflammation. The swelling degree (volume, in mL) of the injected paw was determined with a digital plethysmometer prior to, and 1, 2, 3, 4, and 5h after the administration of the phlogistic agent. Four groups of Wistar rats (both sexes, n=4) randomly selected, each weighing 180-220g, were used. Group 1 served as the negative control and orally received 10mL/kg of distilled water; while Groups 2 and 3 were orally administered with 1000mg/kg of fresh Aloe gel, and 1000 mg/kg of 48-hour (4 °C) refrigerator-stored Aloe gel, respectively; and Group 4 received diclofenac (100mg/kg, orally) as the positive control.
Comparative anti-inflammatory activity of A. schweinfurthii gel and A. vera gel
Carrageenan-induced inflammation test in mice
Anti-inflammatory action of freshly extracted A. schweinfurthii gel and A. vera gel was each determined using male Swiss mice in carrageenan-induced paw oedema anti-inflammatory experiment according to a modified procedure of Bouassida et al.,23 and Gupta & Gaud.24 Four groups of the mice (18-22g in weight), five mice per group (n=5), were randomly selected for the study. Group 1 mice were orally administered with distilled water (10μL/g) as negative control. Groups 2, and 3 orally received: 1000mg/kg of A. schweinfurthii gel, and 1000mg/kg of A. vera gel, respectively; while Group 4 animals received diclofenac (100mg/kg b.w., orally) as the positive control. The baseline right hind paw size (volume, in mL) of each mouse was determined with a digital plethysmometer. Then 0.05mL of 0.1 % w/v carrageenan aqueous solution was injected into the sub-plantar region of the animal’s right hind paw one hour after pre-treatment with each test agent. The size of the injected paw swelling was then determined with plethysmometer 1, 2, 3, 4, and 5h following administration of the inflammogen.
Statistical analysis
The data from antinociceptive activity evaluation (the acetic acid-induced writhing and formalin-induced paw-lick tests) were analyzed with one-way analysis of variance (ANOVA) followed by Dunnett's post-hoc test; while data obtained in the anti-inflammatory (egg albumin and carrageenan induced oedema) experiments were analyzed using two-way ANOVA with Tukey’s post test, on Graph Pad Prism version 5.00 for Windows, Graph Pad Software (San Diego California USA, www.graphpad.com).
Composition of A. schweinfurthii gel
The phytochemical screening for secondary metabolites indicated the presence of carbohydrates and flavonoids in A. schweinfurthii gel; but tannins, sterols and saponins were not detected in the gel. Figure 1 shows the GC-MS chromatogram of the freeze-dried A. schweinfurthii gel indicating the presence of six major compounds eluted with different retention times. Table 1 shows the compounds identified, among which two compounds (phytol and hexanedioic acid) make up 79.28% of the gel.
|
Retention Time (min) |
Compound |
% Composition |
|
29.370 |
Hexanedioic acid (Adipic acid) |
34.88 |
|
Molecular formula: C6H10O4 |
||
|
Molecular weight: 146.14 g |
||
|
25.367 |
Phytol |
44.40 |
|
Molecular formula: C20H40O |
||
|
Molecular weight: 128.17g |
||
|
23.271 |
Oleic acid |
2.70 |
|
Molecular formula: C18H34O2 |
||
|
Molecular weight: 282.47g |
||
|
21.688 |
3-Methyl-1-penten-4-yn-3-ol {Synonym: Ethynyl methyl vinyl carbinol} |
3.53 |
|
Molecular formula: C6H8O |
||
|
Molecular Weight: 96.13g |
||
|
20.957 |
6-Nonen-1-ol |
11.97 |
|
{C2H5CH=CH(CH2)5OH} |
||
|
Molecular formula: C9H18O |
||
|
Molecular Weight: 142.24 g |
||
|
17.954 |
N-(Trifluoroacetyl)-N,O,O',O''-tetrakis(trimethylsilyl)norepinephrine {Synonym: beta,3,4-Tris[(trimethylsilyl)oxy]-N-(trifluoroacetyl)-N-(trimethylsilyl) benzene ethanamine} |
2.53 |
|
Molecular formula: C22H42F3NO4Si4 |
||
|
Molecular Weight: 553.90g |
Table 1 Compounds identified in A. schweinfurthii freeze-dried gel
Comparison of some characteristics of A. schweinfurthii and A. vera gels
Proximate and elemental constituents: The proximate compositions of A. schweinfurthii and A. vera leaf gels were similar. Water constituted the highest percentage of the gels (≈93%), whereas the lipid contents (fats) constituted the lowest percentage composition of both gels; crude fiber was absent (Table 2). Potassium and calcium constituted the highest concentrations of the mineral elements, while manganese was found at the lowest concentration in both gels (Table 3).
|
Components |
Aloe schweinfurthii* |
Aloe vera* |
|
Moisture |
93.48±0.44 |
93.0±0.35 |
|
Protein |
3.18±0.03 |
3.49±0.04 |
|
Ash |
1.75±0.02 |
1.79±0.03 |
|
Carbohydrates |
0.93±0.07 |
0.92±0.06 |
|
Fat/Lipid |
0.67±0.01 |
0.80±0.02 |
|
Fiber |
0.0 |
0.0 |
Table 2 Proximate Composition (%) of Aloe schweinfurthii and Aloe vera gels
*Data values are mean±SEM of triplicate tests.
|
Metallic constituent (symbol) |
Concentration of element determined in gel (mg/L) |
|
|
Aloe schweinfurthii |
Aloe vera |
|
|
Potassium (K) |
587.80 |
403.29 |
|
Calcium (Ca) |
190.70 |
584.02 |
|
Magnesium (Mg) |
37.75 |
90.16 |
|
Sodium (Na) |
73.71 |
48.73 |
|
Iron (Fe) |
4.47 |
3.07 |
|
Copper (Cu) |
3.83 |
2.61 |
|
Manganese (Mn) |
0.78 |
0.36 |
Table 3 Mineral Element Constituents and Their Concentrations in Aloe schweinfurthii and Aloe vera Gels
Physicochemical and antimicrobial properties
Fresh A. schweinfurthii and A. vera gels exhibited comparable density and pH values, but A. schweinfurthii gel demonstrated relatively much lower viscosity value (Table 4).
|
Physicochemical property |
Aloe schweinfurthii gel |
Aloe vera gel* |
|
Density (g/ml) |
0.9850±0.0003 |
0.9945±0.0003 |
|
Viscosity (cP) |
1.47±0.003 |
6.44±0.045 |
|
pH |
5.7±1.5 |
4.5±0.5 |
Table 4 Physicochemical Properties of Aloe schweinfurthii and Aloe vera Gels
*Data values are mean±SEM of triplicate tests.
Acute toxicity study of A. schweinfurthii gel
All the test doses of A. schweinfurthii leaf gel in mice by oral administration, up to 10,000 mg/kg, produced no mortality, signifying that the LD50 was ≥10,000 mg/kg, and therefore not toxic upon acute administration.
Antinociceptive activity of A. schweinfurthii leaf gel
Acetic acid-induced writhing test
All the tested doses of A. schweinfurthii leaf gel (100–1000mg/kg) demonstrated significant reduction (p<0.001) of the mean writhing behaviour in mice compared against the negative control (Figure 2), thus clearly indicating antinociceptive activity of the gel. The writhing pain inhibition levels (26, 36, 38, 39, and 40 %) of the graded A. schweinfurthii gel doses studied (100, 250, 500, 750, and 1000mg/kg, respectively) followed the same trend, even though the effects were not significantly different (p>0.05). Diclofenac (75mg/kg), the positive control, similarly demonstrated significant reduction of the mean writhing (p<0.001) compared to the negative control, and a much greater level of writhing pain inhibition (99 %) than that produced by each of the different A. schweinfurthii gel doses (Figure 2).
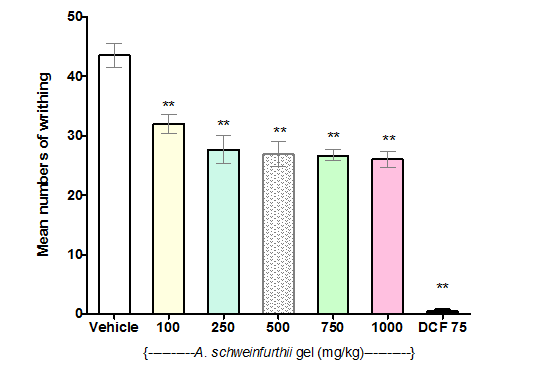
Figure 2 Effect of Aloe schweinfurthii gel on acetic acid-induced writhing in mice.
Each bar represents the mean number of writhing in mice (n=6) that received the indicated Aloe schweinfurthii gel or control dose.
Test group: Vehicle, I.p,; A schweinfurthii gel, p.o; and DCF 75(Diclofenac 75mg/kg) i.p.
Significant difference compared to Vehicle: **=p<0.001
Formalin-induced paw licking test
Antinociceptive activity of A. schweinfurthii leaf gel was evident in the first phase (0 – 5min) of the formalin-induced paw-licking test. At 500 and 750mg/kg doses, the gel significantly (p<0.05, for each dose) reduced paw-lick time in mice compared to the negative control (Figure 3A). Analysis of the results further revealed dose-dependent antinociceptive effects of the gel, from 250 through 750 mg/kg doses; while the antinociceptive action (paw-lick inhibition) demonstrated by 250mg/kg dose of A. schweinfurthii gel was equivalent (p>0.05) to that of acetylsalicylic acid (ASA, 100mg/kg; positive control) (Figure 3A). However in the second phase (15-30 min) of formalin test, paw licking by the animals was not observed (giving a zero score) for all the A. schweinfurthii gel doses (250–750mg/kg) and ASA; and showing therefore a significantly different result (p<0.0001) compared to the negative control group of mice, which demonstrated a mean paw licking time of 6.5 seconds (Figure 3B). The complete inhibition of paw licking at the second phase of the formalin test revealed anti-inflammatory activity of the gel at the tested doses.20,21 The positive control also showed anti-inflammatory activity (Figure 3B).
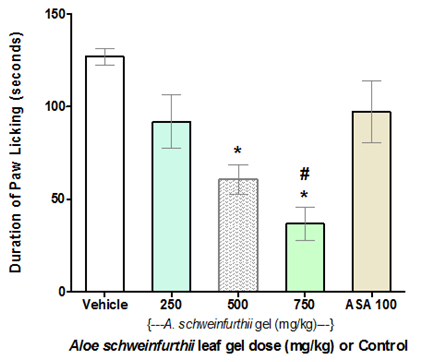
Figure 3A Effect of Aloe schweinfurthii gel on Formalin-induced nociception in mice (Phase 1).
Each bar represents the mean paw-lick duration (seconds) in Phase I testing of mice group (n=6).
Test group: Vehicle, I.p,; A schweinfurthii gel, p.o; and ASA 100(Aspirin 100mg/kg) i.p.
Significant difference (p<0.05):*= Compared to Negative Control; #=Compared to Positive Control.
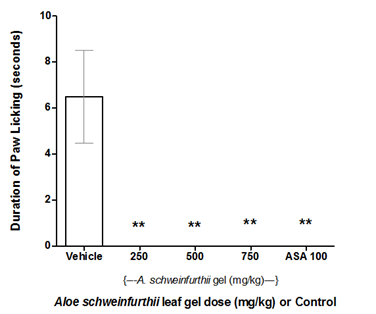
Figure 3B Effect of Aloe schweinfurthii gel on Formalin-induced nociception in mice (Phase 2).
Each bar represents the mean paw-lick duration (seconds) in Phase II testing of mice group (n=6).
Test group: Vehicle, I.p,; A schweinfurthii gel, p.o; and ASA 100(Aspirin 100mg/kg) i.p.
Significant difference (p<0.0001):**= Compared to Negative Control.
Anti-inflammatory activity of A. schweinfurthii leaf gel in rat
A. schweinfurthii gel (freshly extracted) demonstrated anti-inflammatory activity in the egg albumin-induced rat paw-oedema test. The gel (at 1000 mg/kg dose) significantly reduced the mean paw-oedema size (compared to the negative control) at 1 and 2h (T1 and T2; p<0.05 and p<0.01, respectively) following administration of the phlogistic agent (Figure 4). The positive control (diclofenac; 100 mg/kg) also demonstrated significant reduction of the mean rat paw-oedema volume at T2 (p<0.001), compared to the negative control (Figure 4). A. schweinfurthii gel that had been stored in the refrigerator (4˚C) for 2 days, at same 1000 mg/kg dose, similarly caused significant reduction of the rat paw oedema size (compared to the negative control) at T2, T4, and T5 (p<0.001, p<0.01, and p<0.01, respectively); and also produced significant decrease of the mean rat paw oedema, compared to the effect of the fresh gel at T4 and T5 (p<0.05 and p<0.01, respectively) (Figure 4). However, the mean rat paw oedema reductions elicited by the fresh A. schweinfurthii gel or by its 2-day refrigeration-stored sample at 1000mg/kg each, were not significantly different from the paw volume reduction produced by diclofenac (positive control) throughout the duration of the experiments (T1-T5) (p>0.05), suggesting that the activities of the neat gel (fresh or 2-day refrigeration-stored) were generally comparable to that of diclofenac, the positive control (Figure 4).
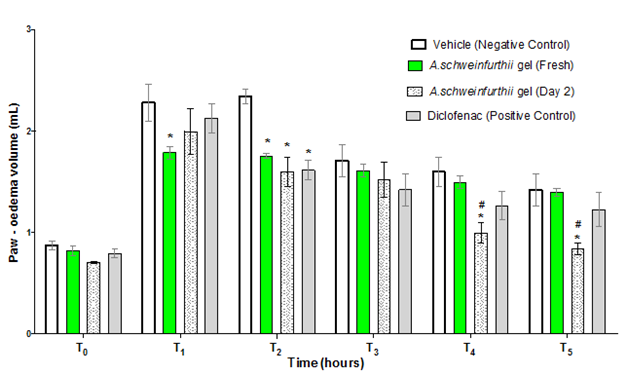
Figure 4 Effect of Aloe schweinfurthii gel on egg Albumin-induced paw oedema in rat.
Each bar represents the mean paw-oedema volume of rats (n=4) that received the indicated Aloe schweinfurthii gel or Control.
T0= zero hour; T1 = first hour; T2 = second hour; T3= third hour; T4= first hour; T5=fifth hour.
Significant difference:*= Compared to Negative Control; #= compared to Fresh A schweinfurthii gel.
Comparative analysis of anti-inflammatory activities of A. schweinfurthii gel and A. vera gel in mice
Figure 5 shows the anti-inflammatory activities of A. schweinfurthii gel and A. vera gel (1000mg/kg dose of each) in mice, which were indicated by significant reductions in the mean paw volume of the test animals (p<0.001) when compared to the negative control, but only at the fourth hour (T4) following administration of the phlogistic agent. At this testing hour (T4), no significant difference was observed (p>0.05) in the mean paw-volume reduction of the animals that received diclofenac compared to those that received the plant gel samples. This suggested that, in the carrageenan-induced mice paw-oedema experimental model, the anti-inflammatory activities of all the test agents including the positive control were relatively equivalent at this point. The results (Figure 5) further revealed an apparent slow onset of action for the neat A. schweinfurthii gel; in that the mean paw-oedema size of the mice, treated with the gel, at the second hour following oedema induction (T2), was still significantly greater (p<0.05) than the mean for the negative control group. Diclofenac (the positive control), on the other hand, showed an earlier onset of action than either of the two aloe gels, by demonstrating significant attenuation of the mice paw oedema (p<0.05; compared to the negative control), which occurred at the third hour (T3), and lasted through the fifth hour (T5) of testing (Figure 5).
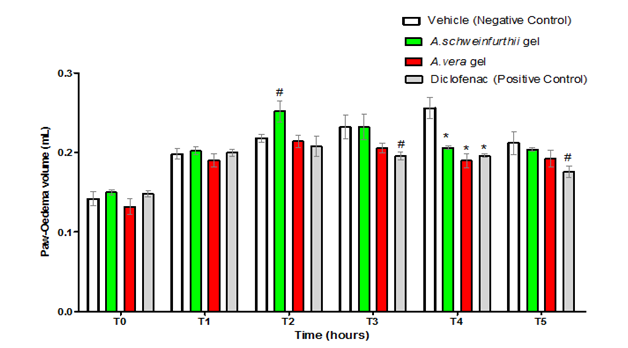
Figure 5 Anti-inflammatory activity of A. schweinfurthii gel compared to controls: A. vera gel and diclofenac.
Each bar represents the mean paw-oedema volume in mice group of rats (n=5) that received the indicated Aloe gel, Formulation or Control.
T0= zero hour; T1 = first hour; T2 = second hour; T3= third hour; T4= first hour; T5=fifth hour
Significant difference compared to Negative Control; #= p<0.05;*=p<0.001.
In this study, we have explored the physicochemical characteristics of A. schweinfurthii leaf gel and determined its composition by GC-MS analysis. The results showed that the gel was similar in many respects to A. vera gel. For example, both Aloe gels qualitatively contained carbohydrates, high moisture contents,25 similar proximate cum mineral/elemental compositions, pH and density values (Tables 2-4). However, some differences in the gel characteristics still exist which could be used as quality control tools for differentiating between their commercial samples or for detecting sample adulteration; e.g. relative higher viscosity of A. vera than A. schweinfurthii. Whereas the yellow sap of the A. schweinfurthii leaf had been earlier reported to produce laxative activities,3 the biological properties of the colourless A. schweinfurthii leaf parenchyma gel were investigated for the first time in this study. We have found the gel to be relatively safe in the acute oral toxicity experiment, and to possess antinociceptive as well as anti-inflammatory activities in mice and rat models.
Some chemical compounds identified in A. schweinfurthii gel, one or more of which may account for its biological activities, include phytol and the flavonoids. On the other hand, the biological activities of A. vera gel including anti-inflammatory effects26 have been mainly attributed to its galactomannan polysaccharide contents working probably in synergy with other compounds.27 However, Añibarro-Ortega et al.28 had listed flavonoids, chromones, anthrones and phenolic acids as major phenolic components in the fillet, mucilage, rind and flower of A. vera, while the GC-MS characterization of tannins derived from A. vera rind extract had similarly shown the presence of phytol (14.40%) along with other compounds.29
The antinociceptive and anti-inflammatory actions of A. schweinfurthii gel found in the present study may be due in large part to its phytol component, coupled or not with the flavonoid compounds. Phytol, present as 44.40% composition of the freeze-dried gel (Table 1), is a natural plant chlorophyll component which has been reported to demonstrate pronounced antinociceptive effect in mice by both central and peripheral actions; significantly reducing contortions in acetic acid-induced writhing test and reducing paw licking time in both neurogenic and inflammatory phases of the formalin test compared to controls.30 The finding is quite similar to the results obtained in the present study (Figures 2&3), where A. schweinfurthii gel produced a dose-dependent decrease of pain stimuli in mice (100–1000 mg/kg), which was comparable to that of aspirin (ASA) but weaker than the effect of diclofenac, the positive controls. Also, Silva et al.31 reported that phytol (at 7.5, 25, 50, and 75mg/kg doses) demonstrated inhibition of acute inflammation and redox-protective pharmacological activities through significantly reducing carrageenan-induced paw oedema in a dose-dependent manner. A. schweinfurthii gel in the present study similarly reduced carrageenan-induced paw oedema (Figure 5). Thus, the analgesic and anti-inflammatory activities exhibited by this gel may be correlated to the presence of phytol, constituting about 44% among other constituents in the gel. Other constituents may also contribute to the overall bioactivity of the gel in this study.
The flavonoid content of A. schweinfurthii gel is another useful phytochemical constituent of significance in further explaining its biological activities as observed in this study. Flavonoids are ubiquitous, naturally-occurring polyphenolic compounds as essential secondary metabolites in plants, which exhibit different biological effects including anti-inflammatory, antioxidant and antimicrobial, which have been reported both as in vitro and in vivo studies.32 They have also demonstrated inhibition to regulatory enzymes or transcription factors important for controlling mediators involved in inflammation, thereby functioning as potent antioxidants with potential to attenuate tissue damage or fibrosis.33 Numerous studies in animal models have shown flavonoids as capable of inhibiting both the onset and progression of inflammatory diseases.34
The present study has clearly demonstrated anti-inflammatory activities of A. schweinfurthii leaf gel at 1000 mg/kg dose, orally administered to both rats and mice models in the egg-albumin and carrageenan-induced oedema tests, respectively (Figure 4 & 5), using the usual standard animal experimental protocols of Gregory et al.35 and Zhao et al.22 The 2-day refrigerator-preserved A. schweinfurthii gel also exhibited anti-inflammatory potency comparable to that of the fresh gel and to diclofenac, the positive control (Figure 4). Anti-inflammatory efficacy of the 2-day refrigerator-stored gel was evaluated in this study in anticipation of storage concerns in its future use. Processing techniques, besides refrigeration, toward stabilizing A. vera gel and minimizing its decomposition due to natural enzymatic reactions and bacterial growth due to the presence of oxygen have been described.36 However, refrigeration is simple and a common storage option that could extend shelf-life stability and hence, sustain efficacy of the fresh A. schweinfurthii gel.
From the results obtained in this study, it is concluded that A. schweinfurthii leaf gel is non-toxic orally while demonstrating significant analgesic and anti-inflammatory potentials comparable to A. vera gel and standard drugs. Furthermore, the major phytochemical constituent identified by GC/MS in the gel was phytol.
None.
Author declares that there are no conflicts of interest.
None.

©2021 Oyesomi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.