International Journal of
eISSN: 2381-1803


Background: Dental caries is a major public health concern worldwide. In this paper, we investigated the antimicrobial activity of three synthetic monoketone curcuminoids (MKCs) against a representative panel of cariogenic bacteria and examined some structure-antimicrobial activity relationships of MKCs.
Methods: MKCs1 (curcumin A) and 2 were obtained by Claisen-Schmidt condensation in acidic conditions. Compounds 1a and 2awere obtained from 1by catalytic hydrogenation. The minimum inhibitory concentration (MIC) values of 1a, 1b, and 2 were determined by using the broth micro dilution method in 96-well microplates. Chlorhexidine was used as positive control.
Results: Compound 2afforded the lowest MIC values against Streptococcus mutans (MIC=50μg/mL) and Streptococcus mitis (MIC=50μg/mL), as well as moderate activity against S. sanguinis (MIC=100μg/mL), S. salivarus (MIC=200μg/mL). These results revealed that the antimicrobial activity of MKCs is enhaced by the presence of a hydroxy group at the aromatic rings, as well as the carbonyl group at C1 and the double bonds between C2-C3 and C2’-C3’.
Conclusion: Compounds 1 and 2 displays promising antimicrobial activity against some cariogenic bacteria. Our results suggest that these compounds might be promising for the development of new oral care products.
Keywords: antibacterial activity, dental caries, monoketone curcuminoids, oral pathogens, Streptococcus mutans
MKCs, monoketone curcuminoids
Dental caries constitutes a major public health concern worldwide. This pathology is caused by acidogenic and aciduric bacteria, which produce a structurally and functionally organized biofilm (dental plaque)on the tooth surface.1 Streptococcus mutansis one of the most important colony-forming bacteria present in the bucal microbiota, stands out due its ability of producing substances that favor adhesion and the accumulation of other microorganisms, forming a resistant extracellular matrixthat can destroy dental hard tissue.2Brushing and flossing the teeth to remove dental plaque, as well as conducting periodic dental cleaning or prophylaxis are the most efficient ways to prevent caries. However, most people fail to maintain an efficient biofilm control through mechanical removal only, which has increased the use of oral products containing antimicrobial agents to diminish biofilm formation on the tooth surface.3 Although chlorhexidine is currently the most effective anti plaque agent, its use have been recommended by dentists only for short periods due to reversible local side effects.4
Recently, monoketone curcuminoids (MKCs), or 1,5-diarylpentadien-3-one derivatives, have been reported as promising compounds with respect to their antimicrobial activity against a panel of cariogenic bacteria, including S. Mutans.5 Although some preliminary structure-relationships have been previously proposed, the effect of some important structure features on the antimicrobial activity was not evidenced. In this paper, we report on the antimicrobial activity of three MKCs derivatives against cariogenic bacteria aiming to better understand the structure-antimicrobial relationships among these compounds.
Synthesis of monoketone curcuminoids 1-2, 1a-1b
Compounds1and 2(Scheme 1) were obtained by aldol condensation in acidic conditions, as previously reported.6 Briefly, a mixture of acetone (580mg, 10mmol) and vanillin(20mmol) for compound 1 or 4-hidroxy-benzaldehyde (20mmol) for 2was slowly added to acetic acid (50mL) saturated with hydrogen chloride at O oC. The reaction mixture was stirred at room temperature for 24h. Next, the crude reaction mixture was poured into ice-cold water (200mL) and then extracted with ethyl acetate (3x30mL). After drying the organic portion over MgSO4, filtration, and solvent removal under reduced pressure, the resulting solid was purified using silica gel flash column chromatography using hexane: ethyl acetate 1:1 (v/v) (Figure 1). Compounds 1aand1b were obtained from 1 by catalytic hydrogenation. In this procedure, compound 1 dissolved in HPLC grade ethyl acetate was added to a high-pressure reactor together with Pd/C (10%) and kept at room temperature under stirring, H2 atmosphere, and pressure of 100 psi for 18h.7 Chemical structures of compounds 1, 1a, 1b, and 2a were confirmed on the basis of NMR, IR, and MS analyses.
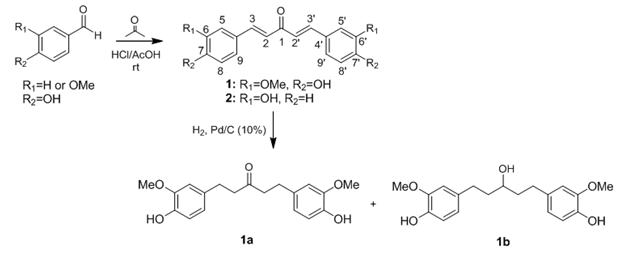
Figure 1 Reaction conditions for the synthesis of compounds 1, 2 and 1a-1b usingaromatic aldehydes and acetone, r.t., and catalytic hydrogenation with H2, Pd/C (10%), 100psi, r.t.
(1E,4E)-1,5-bis (4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one (1): Yield 21.0%, orange powder. IR (KBr pellet) of 1(C19H18O5). νmax/cm-1: 3411 (nOH), 3005 (nCH), 1714 (nC=O), 1589 (nC=C), 1093 (nC-O). LRESI-MS (m/z, %relative intensity): 327(100) [M+H]+. NMR 1H (400MHz, CDCl3): δ 3.95 (6 H, s, H10=H10’), 6.93 (2 H, d, J2,3=2’,3’=15.8Hz, H2=H2’), 6.94 (2 H, d, J6,5=6’,5’=8.2Hz, H6=H6’), 7.11 (2 H, d, J9,5=9’,5’=1.4Hz, H9=H9’), 7.17 (2 H, dd, J5,6=5’,6’=8.2, J5,9=5’,9’=1.4Hz, H5=H5’), 7.67 (2 H, d, J3,2=3’,2’=15.8Hz, H3=H3’), 7.67 (2 H, d, J3,2=3’,2’=15.8Hz, H3=H3’). 13C (100MHz, CDCl3): δ 56.2 (CH3, C10=C10’), 109.8 (CH, C9=C9’), 115.0 (CH, C6=C6’), 123.4 (CH, C5=C5’), 123.5 (CH, C2=C2’), 127.6 (C, C4=C4’), 143.3 (CH, C3=C3’), 146.9 (C, C7=C7’), 189.2 (C=O, C1).
1,5-bis(4-hydroxy-3-methoxyphenyl)pentan-3-one (1a): Yield 37%,white powder. IR (KBr pellet) of 1a (C19H22O5). νmax/cm-1:3520 (n-OH), 2925 (nCsp2-H), 1701 (nC=O), 2999 (nC-H), 1232 (nC-O). LRESI-MS (m/z, %relative intensity): 331 (100) [M+H]+. NMR 1H (400 MHz, CDCl3): δ2.66 (4 H, t, J2,3=2’,3’= 7.4Hz, H2= H2’), 2.81 (4H, t, J3,2=2’,3’= 7.4 Hz, H3=H3’), 3.85 (6H, s, H10=H10’),6.62 (2 H, d, J9,5;9’,5’=1.9 Hz,H9=H9’), 6.65 (2 H, dd, J5,6;5’,6’=7.9Hz and J5,9;5’,9’=1.9Hz, H5=H5’), 6.60 (2H, d, J6,5;6’,5’=7.9, Hz,H6=H6’).13C (100 MHz, CDCl3):δ 29.6 (CH2, C3=C3’), 45.0 (CH2, C2=C2’), 55.9 (CH3, C10=C10’), 111.1 (CH, C9=C9’), 114.4 (CH, C6=C6’), 120.8 (CH, C5=C5’), 133.0 (C, C4=C4’), 144.0 (C, C7=C7’), 146.6 (C, C8=C8’), 209.7 (C=O, C1).
4,4'-(3-hydroxypentane-1,5-diyl)bis(2-methoxyphenol) (1b):35.3%Yield , white powder. IR (KBr pellet) of 1b(C19H24O5). νmax/cm-1:3409 (nOH), 2913 (nCsp2-H), 1513 (nC-O), 2848 (nC-H), 1240 (nC-O). LRESI-MS (m/z, %relative intensity): 333(100) [M+H]+. NMR 1H (400 MHz, CDCl3):δ1.74 (2 H, dddd, J2a,2b;2a’,2b’=13.8 Hz, J2a,3b;2a’,3b’=9.5Hz, J2a,1;2a’,1=7.7Hz, J2a,3a;2a’,3a’=6.0Hz, H2a,2a’), 1.80 (2 H, dddd, J2b,2a=2b’,2a’=13.8 Hz, J2b,3a=2b’,3a’=9.4 Hz, J2b,3b=2b’,3b’=6.8Hz, J2b,1=2b’,1=4.5Hz, H2b,2b’), 2.61 (2 H, ddd, J3b,3a=3b’,3a’=13.8Hz, J3b,2a=3b’,2a’=9.3Hz, J3b,2b=3b’,2b’=6.8Hz, H3b,3b’), 2.71 (2 H, ddd, J3a,3b=3a’,3b’= 13.8Hz, J3a,2b=3a’,2b’=9.2, J3a,2a=3a’,2a’=6.5Hz, H3a,3a’), 3.67 (1H, dt, J1,2a=1’,2a’=7.7Hz, J1,2b=1’,2b’= 4.5Hz, H1), 3.84 (6H, s, H10=H10’), 3.68 (2H, dd, J5,6;5’,6’=8.0Hz and J5,9;5’,9’=1.8Hz, H5=H5’), 6.64 (2H, d, J9,5;9’,5’=1.8Hz, H9=H9’), 6.82 (2H, d, J6,5;6’,5’=8.0Hz, H6=H6’). 13C (100MHz, CDCl3): δ 32.0 (CH2, C3=C3’), 39.3 (CH2, C2=C2’), 56.1 (CH3, C10=C10’), 71.0 (CH, C1=C1’), 111.1 (CH, C9=C9’), 114.4 (CH, C6=C6’), 120.9 (CH, C5=C5’), 134.0 (C, C4=C4’), 143.7 (C, C7=C7’), 146.5 (C, C8=C8’).
(1E,4E)-1,5-bis(4-hydroxyphenyl)penta-1,4-dien-3-one (2): Yield 43.8%, orange powder. IR (KBr pellet) of 2 (C17H14O3). νmax/cm-1: 3325(nOH), 2924(nCsp2-H), 1596(nC=O), 1511(nC=C).LRESI-MS: (m/z, %relative intensity): 267(100) [M+H]+. NMR 1H (400 MHz, CDCl3):δ6.90 (4H, d, J6,5;6’,5’=8.5Hz, H6=H6’=H8=H8’), 7.10 (2 H, d,J2,3=2’,3’=16.0Hz, H2=H2’), 7.60 (4 H, d,J5,6=5’,6’=8.5Hz, H5=H5’=H9=H9’), 7.70 (2 H, d,J3,2=3’,2’=16.0Hz, H3=H3’).13C (100MHz, CDCl3): δ 116.6 (CH, C5=C5’=C9=C9’), 123.6 (CH, C2=C2’), 127.4 (C, C4=C4’), 130.9 (CH, C6=C6’=C8=C8’), 142.9 (CH, C3=C3’), 160.5 (C, C7=C7’), 188.8 (C=O, C1).
Antimicrobial assays
The in vitro antimicrobial activity of compounds1-2, 1a-1b was evaluated on the basis of their minimum inhibitory concentration (MIC) values, according to the previously reported methodology.8 Briefly, Streptococcus mitis (ATCC 49456), Streptococcus mutans (ATCC 25175), Streptococcus salivarius (ATCC 25975), Streptococcus sobrinus (ATCC 33478), Streptococcus sanguinis (ATCC 10556), Lactobacillus casei (ATCC 11578), and Enterococcus facials (ATCC 4082) were tested by the broth micro dilution method, in 96-well micro plates. Colonies of the selected bacteria were cultured in blood agar (Difco Labs, Detroit, MI, USA) at 37 oC for 24h Next, the inoculum quantity was standardized to match 0.5 in the McFarland scale (1.5x108 CFU/mL) using a spectrophotometer Femto (São Paulo, Brazil) at 625nm. The microorganism suspensions were diluted to a final concentration of 5x105CFU/mL. Compounds 1, 1a, 1b, and 2 were dissolved in tryptic soy broth (TSB, Difco) and dimethylsulfoxide (DMSO) (Merck, Darmstadt, Germany) to achieve final concentrations between0.195 and 400μg/mL. Inoculated micro plate wells containing TSB (1:5 v/v and 100%) and DMSO (1%) were usedas negative control. To ensure medium sterility, anon-inoculated well was also added. Chlorhexidine dihydrochloride (Sigma-Aldrich, St. Louis), which was used as positive control was dissolved in TSB and tested at concentrations from 0.115 to 59.0μg/mL. The microplates were then sealed with plastic film and incubated for 24 that 37°C. Next, 30μL of revealing 0.02% resazurin (Sigma-Aldrich, St. Louis) was added to each microplate well, to indicate the microbial viability. We conducted the experiments in three replicates for each microorganism. The MIC values were assessed on the basis of the MCKs capacity to prevent the resazurin color solution from changing.
In a previous work, we reported the antimicrobial activity of twenty-five monoketone curcuminoids, including curcumin A (1), which was demonstrated to be more effective than curcuminagainst S. mutans and S. mitis. However, some important structure-antimicrobial relationship structure of MKCs were not clear. Here, we investigated the antimicrobial activity of three MKCs against a panel of cariogenic bacterial strains on the basis of their minimum inhibitory concentration (MIC) values.
Classification of the antibacterial activity was based on MIC values, in this way, MIC values lower than 100mg/mL, between 100< MIC<500mg/mL or between 500<MIC<1000mg/mL and higher than 1000mg/mL correspond to promising, moderate, weak and inactive activities, respectively.9–12 In this way, according to these criteria, compounds 1 and 2 displayed the lowest MIC values against S. mutans (MIC=50μg/mL) and S. mitis (MIC=50μg/mL), as well as moderate activity against S. sanguinis (MIC=100μg/mL), S. salivarus (MIC=200μg/mL) and compound 1 was also active against L. casei(MIC=100μg/mL). Compound 2 also had promising activity for L. casei (MIC=25μg/mL) and moderate activity against S. sobrinus(MIC=200μg/mL). Moreover, the compounds 1b also displayed moderate active against S. mutans ((MIC=200μg/mL), S. mitis (MIC=100μg/mL), S. sanguinis (MIC=200μg/mL) and L. casei (MIC=200μg/mL). The activity of compound 1 and 2 against S. mutans is a note worthy result because natural compounds capable to inhibit this primary causative agents of dental cariesare still scarce.2 None of the tested MKCs was significantly active against Enterococcus faecalis (Table 1).
Microorganism |
1 |
1a |
1b |
2 |
Positive control |
Streptococcus mutans(ATCC 25175) |
50 |
>400 |
200 |
50 |
0.92b |
Streptococcus mitis(ATCC 49456) |
50 |
400 |
100 |
50 |
1.84b |
Streptococcus salivarius(ATCC 25975) |
200 |
>400 |
>400 |
200 |
1.84b |
Streptococcus sanguinis(ATCC 10556) |
100 |
400 |
200 |
100 |
0.92b |
Streptococcus sobrinus(ATCC 33478) |
400 |
400 |
400 |
200 |
0.92b |
Enterococcus faecalis(ATCC 4082) |
>400 |
>400 |
>400 |
400 |
7.37b |
Lactobacillus casei(ATCC 11578) |
100 |
400 |
200 |
25 |
0,92b |
Table 1 Minimum inhibitory concentration (MIC) values [μg/ml] of the monoketone curcuminoids against selected cariogenic bacteria
bChlorhexidinedihydrochloride
According to previous reports, MKCs bearing a double bond between C2 and C3, and between C2 'and C3' were inactive against the tested microorganisms in the range of concentrations tested, except for compound1 (curcumin A).5 These results led us to suspect that the higher activity observed for curcumin A could be related to the simultaneous presence of methoxy and hydroxy groups in the aromatic ring, since compounds displaying one, two or three methoxy groups at the aromatic ring were inactive.5 To verify this hypothesis, we synthesized the curcumin A derivatives 1a and 1band the hydroxy curcuminoid 2 and evaluated their antimicrobial activity against cariogenic bacteria. Comparison between the MIC values of curcumin A (1) and compounds 1a and 1brevealed that absence of the double bond between reduction of the carbonyl group at C1 and/or the double bond between C2 and C3/C2’-C3’ caused a significative reduction in the antimicrobial activity. In addition, it was observed that compound 2, which does not have a methoxy group in its structure displayed antimicrobial activity very similar to that of compound 1. Compound 2 was more activity than curcumin A against S. sobrinus (MIC=200μg/mL) and L. casei (MIC=25 μg/mL). These results revealed that the hydroxyl group at the aromatic ring plays a key role for the antimicrobial activity, and its presence is more important for this activity than the methoxyl group.
Data obtained from the present study, in combination with those previously reported, revealed that the carbonyl group at C1and the double bonds between C2 and C3 are important structure features for the antimicrobial activities of MKCs. However, the nature of the substituent at the aromatic rings plays an important role in this activity. Among the tested compounds, compounds displaying a hydroxyl group (1 and 2) were much more active than those with methoxyl, bromine, chlorine, and methyl groups.5
Compounds 1 and 2 display promising antimicrobial activity against Streptococcus mutans, which is one of the main causative microorganismof dental caries. The hydroxyl group attached to the aromatic rings, as well as the double bond between C2-C3 and C2C3’ and the carbonyl group at C1, are the most important structure features for the antimicrobial activity. Taken together, these results suggest that these compounds might be promising for the development of new oral care products.
The authors thank the Brazilian foundations FAPESP (Proc. FAPESP 2016/19272-9 and 2013/20094-0) for the financial support and fellowships.
The authors declare no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.