International Journal of
eISSN: 2574-9862


Literature Review Volume 7 Issue 1
1Chelia District Agricultural office, Gedo Veterinary clinic, Chelia District,West Shewa Zone, Oromia, Ethiopia
2Meta Robi District Agricultural office, Shino Veterinary Clinic, West Shewa Zone,Oromia, Ethiopia
Correspondence: Dr. Tagesu Abdisa Serbessa, Ambo University, School of Graduate Studies, Guder Mamo Mezemir Campus, Department of Veterinary Epidemiology, Ethiopia, Tel +251912110738
Received: December 26, 2022 | Published: April 24, 2023
Citation: Serbessa TA, Geleta YG, Terfa IO. Review on diseases and health management of poultry and swine. Int J Avian & Wildlife Biol. 2023;7(1):27-38. DOI: 10.15406/ijawb.2023.07.00187
Poultry and swine production play an important role in countries' socioeconomic development by providing proteins that support food and nutrition security. However, several infectious and non-infectious diseases hinder the production of swine and poultry. Therefore, this review aim to provide highlight of the common disease and health management of poultry and swine. Poultry production has suffered from different pathogenic microorganisms that cause devastating economic losses in poultry industries worldwide. Poultry can be infected with common diseases like endoparasites, ectoparasites, infectious bronchitis, Marek's disease, fowl cholera, salmonellosis, infectious coryza, fowl pox, avian encephalomyelitis, etc. Health management is a system of preventive medicine that considers the whole poultry farms and the total influences, including social, with respect to relationships with others in the flock, psychological, and environmental factors that affect health. Swine production can be destructed by the influence of infectious diseases, which include Mycoplasma Hyopneumoniae, Actinobacillus pleuropneumoniae, Porcine Reproductive and Respiratory Syndrome Virus, Trichinella spp., Toxoplasma gondii, Salmonella spp., Campylobacter, and Leprospira. This all causes respiratory problems, leg problems, reproductive disorders, gastrointestinal problems, claw and skin problems, parasitic infections, and piglet mortality. Endoparasites and ectoparasites are regarded as the most significant constraints for welfare and health, as well as economic loss, in swine production, particularly during the post-weaning period. However, health management of swine production can reduce the effect of disease and optimize their productivity. Herd health management practices include vaccination, genetic improvement, and observation for all animals’ clinical signs, record keeping, detection and treatment of injury, sanitation, disease, pest control, and animal handlers. Generally, disease has a great risk to the health of poultry and swine animals that causes a decrement in their production. Therefore, the health management on poultry and swine farms should have to be strictly measured and further studies need to be conducted to solve the major problem of economic loss of production.
Keywords: biosecurity, disease, health, management, poultry, swine, vaccination
AMs, antimicrobials; APEC, avian pathogenic escherichia coli; APMV-1, avian paramyxovirus 1; ETEC, enterotoxigenic E. coli; FBD, food borne disease; HB1, hitchener B1; HVT, turkey herpesvirus; MD, marek’s disease; MDV, marek’s disease virus; ND, newcastle disease; NDV, newcastle disease virus; OIE, organization international de epizootic; PRRSV, porcine respiratory and reproductive syndrome virus; PWDS, post weaning diarrhea syndrome
Livestock production is one of the country's economic growth initiatives. Agricultural development was the foundation for the country's economic development. Livestock, poultry, fisheries, and bee production are all used in livestock development strategies. Poultry and swine farming are the finest options for developing countries, especially for low-income investors. Poultry and swine production contribute significantly to a country's socioeconomic growth by delivering proteins that help to ensure food security.1,2 Chicken production can be done on a farm or in a backyard because poultry produces the cheapest protein and eggs, making it possible for humans to consume cheap protein. Swine and poultry byproducts also contribute significantly to economic development, particularly in poor countries. Poultry farming provides excellent opportunity for low-income women and men.1 Pig farming is the fastest-growing livestock industry in the world, and this trend is anticipated to continue in the future years. Due of their high fertility and feed conversion efficiency, pigs have a lower social rank than cattle. Pig production has a number of advantages over other livestock husbandry, including early maturity, a short generation interval, a relatively modest space demand, and the ability to produce maximally under a variety of management settings. It is thought to help with animal protein deficit and is seen as a technique for fighting poverty in the tropics.3–5 Pig production expansion contributes to national growth domestic product and overall economic growth by providing an extra animal protein source for human consumption, creating jobs, and alleviating poverty.6 Large intensive swine farms are not as developed in Ethiopia as they are in other countries, but small-scale pig production as an agricultural activity has only lately been introduced throughout the country.7 Pig farming is characterized in many rural areas of Ethiopia by vast production methods in which animals are allowed to scavenge at household and municipal rubbish disposal sites.8 However, the production of swine and poultry may be decreased with the influence of poor management, including lack of proper health management, inadequate nutritional feeding and inappropriate housing.9,10 Growth, commercialization, profitability, and sustainability of poultry business activities are found to be severely constrained due to diseases.11 The most common infectious and non-infectious diseases in poultry are economically important and a threat to public health. In Ethiopia, the most common infectious diseases that cause failure in poultry production have been reported. The most common diseases include Newcastle diseases, infectious bursal diseases, marks diseases, Pasteurella infections, mycoplasma, coccidiosis, endoparasites, and ectoparasitic diseases.9,12
As the research shows that in small-scale poultry farms susceptible to parasitic diseases, coccidiosis was reported as the most common devastating disease followed by viral diseases.13–15 According to Hailu Newcastle disease, infectious bursal disease, and Marek’s disease are among the major viral diseases of chickens in Ethiopia.16 The most common symptoms of poultry diseases in small-scale poultry farms include depression, ruffled feathers, diarrhea (watery, bloody), panting (respiratory rales), coughing, drooling saliva, swelling of the head and eyes, torticollis and loss of egg production.14 The most common infectious diseases in swine farm production can be categorized into the stages of production as pre-weaning, growing, finishing, and breeding stages.17 The research noticed that the major diseases that affect pig production are classic swine fever, African swine fever, porcine cysticercosis, meningitis, pneumonia, exudative dermatitis, coccidiosis, respiratory diseases, swine dysentery, mastitis, porcine parvovirus, and swine fever, foot and mouth disease, asthma, enteritis, diarrhea, and atrophic rhinitis.18,19 Diseases and injuries are important elements when monitoring health management that characterized by diarrhea (watery, bloody), skin lesions (diamond shape), lameness, leg lesions, encephalomyelitis, depression, excessive salivation, vaginal discharge, frequent coughing, swollen joints, scouring, loss of feed intake, loss of body condition, and the presence of external parasites.20 Poultry and swine production can be improved with the appropriate management of farm housing and rearing environments. Biosecurity and vaccination are the most common health protocols that improve the health and production of swine and poultry in developing countries. New advances in treatments are adding to the collection of modalities to manage animal health.21 In modern systems, temperature, ventilation, feed delivery, water delivery, and sanitation are controlled, often automatically. These enhancements to keep pigs comfortable and stress-free also provide the optimum environment for their immune systems. The technologies developed a system that has helped to protect swine from climate extremes and have offered added enhancements in terms of biosecurity and production. Generally, disease can be reduced by proper sanitation on the farm, biosecurity measures, and vaccination of the chickens.22,23
Even if the health management methods are mentioned and reported, the production of swine and poultry remains in question. Many researchers have reported risk factors for swine and poultry production declines in developing countries. Despite the fact that the health management approach to the production farm has not been conducted in detail concerned with appropriate diseases. Therefore, this paper aim to provide a highlight of the animal disease and health management of swine and poultry.
The Common Poultry Diseases
Poultry production has suffered from different pathogenic microorganisms that cause devastating economic losses in poultry industries worldwide. The diseases caused by pathogens are contagious or infectious diseases because they can be passed from poultry to poultry via direct and indirect routes. According to their biological nature, pathogenic microorganisms which cause diseases can be classified into viruses, mycoplasma, bacteria, fungi, protozoa, and parasites.22, 24,25 According to prevalence, transmission, zoonotic potential, morbidity, and mortality properties, the OIE makes and updates a list of diseases for the poultry industry to monitor. Therefore, the most common poultry diseases as per the list reported by OIE include avian influenza (high or low pathogenicity), Newcastle disease, avian mycoplasmosis, avian infectious laryngotracheitis, avian infectious bronchitis, fowl typhoid, infectious bursal disease, Marek’s disease, infectious coryza, pullorum disease, and coccidiosis.26,27
Major bacterial disease
Bacterial diseases are one of the major effects on the health of poultry production. Salmonellosis, fowl cholera, mycoplasmosis, and colibacillosis are the most threatened diseases. The bacteria can immediately have a direct or indirect influence on producers or farmers. Bacterial diseases have various impacts on poultry production that including: productivity losses (production losses, cost of treatment, market disturbances), loss of income from activities using poultry resources, zoonotic impact and cost expenditure on prevention and control.28
Infectious corza: Hemophilus paragallinarum causes an acute, severe respiratory illness in chickens.29 Birds are the disease's principal reservoir hosts, and they operate as carriers, allowing infection to spread through direct contact, airborne droplets, and fomites.30 The common clinical indications of infectious coryza includes nasal and ocular discharge, swelling around the eyes and cheeks rhinitis, sinusitis, anorexia and reduce egg production in layer flocks.31This disease can be avoided by using immunizations and proper biosecurity practices to defend against the spread of bacteria.32 Avibacterium paragallinarum is naturally found in chickens. The agent is spread between animals by secretions and excretions. Transmission can also happen through the interchange of farm machinery and equipment, as well as employees.33,34
Salmonellosis: Salmonellosis is the most frequent disease in chicken productions, caused by several strains of Salmonella.35 Young birds are susceptible to pullorum disease, while adult fowl are susceptible to fowl typhoid. Salmonella gallinarum is spread through hatcheries, feed, and poultry houses. Salmonella pullorum transmission can occur within 48 hours of hatching, resulting in a decreased rate of shell penetration and feed contamination.36
Salmonella gallinurum causes fowl typhoid, which is more common during the growth season and in mature flocks. The most prevalent clinical manifestations include symptoms that resemble septicemia, in appetence, and death. Another condition caused by Salmonella pullorum is Pullorum disease. Pullorum sickness manifests itself in young chickens as a variety of clinical symptoms. White diarrhea and despair are two symptoms of pullorum sickness.37,38 Salmonellosis can be transferred through poultry farms by contaminated day-old chicks, domestic animals, humans, equipment, water, and feed, as well as from the environment.39 It can also be spread through direct, indirect, and wound infection with an infected bird, as well as through trans-ovarian egg transmission.40 Infection prevention and control programs aim to protect birds from salmonella and manage their health, as well as ensure customer safety and increase the poultry production chain's reliability.41
Mycoplasmas: When Mycoplasma biovars gallisepticum is combined with E. coli, it causes chronic respiratory disease in hens, which can lead to economic consequences.42 Chicks born from infected animals' eggs play an important part in lateral transmission. The most important method of transmission is through eggs. Vertical transmission is detected through contaminated eggs.43 Mycoplasma infections are caused by Mycoplasma synoviae and Mycoplasma gallisepticum, and hens are natural hosts for both. Chronic respiratory infections are caused by Mycoplasma gallisepticum. Coughing, panting, a modest opening of the beak, and a reduction in feed intake are the most common signs. Clinically, there is a decrease in egg production, corneal and conjunctival irritation, face edema, ocular discharge, and it cause the eggshell to thin out, lose its opacity, and become rough. As a result, eggs are more likely to crack or break that causes eggs to be unfertilized as well as decrease in egg production.44
Fowl cholera:This is a septicemic disease of domestic and wild fowl with high mortality and morbidity rates, caused by Pasteurella multocida of the Pasteurellaceae family.45 Adult chickens are more susceptible to this disease than young fowl, and broilers are more resistant to the disease than layers, resulting in deaths at higher rates in laying hens .46 The diseases particularly transmitted through the feces or oral/nasal discharge of animals that have recovered from the infection. Fowl cholera shows some clinical characteristics such as respiratory rales, coughing, and nasal discharge.34
Colibacillosis: This is a disease that is characterized by colisepticemia, hemorrhagic septicemia, coligranuloma, air sac disease, swollen head syndrome, venereal colibacillosis, cellulitis, peritonitis, salpingitis, osteomyelitis, yolk sac infection, and enteritis caused by the avian pathogenic Escherichia coli (APEC) of the Enterobacteriaceae family. Escherichia coli (E. coli) is considered a member of the normal microflora of all warm-blooded animals, including poultry.28 Though they are "non-pathogenic," found in the gastro-intestinal, strains of E. coli are opportunistic when in debilitated or immune suppressed hosts, or when gastro-intestinal barriers are violated, causing infection to poultry, humans, and animals. Moreover, there are certain E. coli strains designated as avian pathogenic E. coli that spread into various internal organs and cause colibacillosis characterized by systemic fatal disease.47 Many opportunistic diseases associated with E. coli in poultry have been reported, including yolk sac infection, omphalitis, respiratory tract infection, septicemia, polyserositis, enteritis, cellulitis, and salpingitis.48
Clostridium perfirnges: Clostridium infects chickens and causes two types of food-borne disease (FBD): intoxication and infection. The intoxication is related to the toxin produced and causes food poisoning, while infection is caused when an adequate dose of food containing the pathogen is ingested.49
Newcastle disease: Newcastle disease (ND) is caused by a group of closely related viruses that form the avian paramyxovirus type 1 (APMV-1) serotype. It is a highly contagious and the most dreaded disease of chickens, turkeys, and many other birds.50,51 Based on the disease produced in chickens under laboratory conditions, NDVs have been placed into five pathotypes such as: apathogenic strains, lentogenic strains, mesogenic strains, viscerotropic velogenic strains and neurotropic velogenic strains. Viscerotropic velogenic NDVs cause a highly severe form of the disease in which hemorrhagic lesions are characteristically present in the intestinal tract. Neurotropic velogenic NDVs cause high mortality following respiratory and nervous signs. Mesogenic NDVs cause respiratory and sometimes nervous signs with low mortality. Lentogenic respiratory NDVs cause mild or in apparent respiratory infection; and asymptomatic enteric NDVs cause in apparent enteric infection.50,52,53 The most common mode of infection is through the oral route. Conjunctival and respiratory routes may also be involved.52,53
Marek’s disease: It is caused by cell associated lymphotopic herpesvirus. Due to its lymphotropic nature, MD virus (MDV) was originally classified in the family Herpesviridae as a member of the subfamily Gammaherpesvirinae.54 However, on the basis of genomic organization, MDV is currently classified with the viruses of subfamily Alphaherpesvirnae. Three serotypes of MDV and related herpes viruses have been defined. Serotype 1 includes all the pathogenic or oncogenic strains of these viruses. Serotype 2 includes naturally non-attenuated strains of MDV. Serotype 3 includes turkey herpesvirus (HVT), the non-oncogenic MDV-related virus isolated from turkey. Marek’s diseases are most commonly affects brachial and sciatic plexus and neve trucks, celiac plexus, abdominal vagus and intercostal nerves. The enlargement of one or more peripheral nerves causes the paralysis of chickens.55
Parasitic disease
Parasitic infections are one of the real issues that generate economic bias in animal farms and rural areas that grow chickens on a regular basis. The most prevalent parasite illnesses in poultry can be split into ectoparasites and endoparasites. Arthropods such as lice, mites, fleas, and ticks, which are isolated from skin and feathers, are examples of external parasites. Protozoa, cestodes, nematodes, and trematodes are internal parasites that are isolated from the digestive tract, blood, and pooled poultry droppings.56 Chickens are very susceptible to infection by a range of intestinal helminths, resulting in significant commercial losses due to tampering with fit development in the late-growth system.57
Cestodes: The cestodes are known to interfere with the metabolisms of certain compounds: they absorb glucose and galactose as well as absorbing amino acids, polypeptides, and proteins. The common cestode that infest chickens are includes: Raillietina tetragona, R. echinobothrida, R. cesticillus, Choanotaenia infundibulum, and Hymenolepis carioca, of which R. cesticillus was the least prevalent. The clinical signs include loss of ruffled feathers, drooping wings, decreased egg production, although with less pathogenic species, the only signs will be poor growth, weight loss, paralysis, leg weakness, and a sudden rise in mean mortality. The eggs of cestodes are observable as white pellets struck in their feces.58,59
Nematodes: Nematode is a common parasite of poultry that reduces productivity. The most prevalent nematodes in poultry include Ascaridia galli, Heterakis gallinarum, Gongylonema ingluvicola, and Syngamus trachea, of which only A. galli and H. gallinarum are most prevalent and the others are rare.60 These helminths affect the metabolism of the host, causing low feed utilization, thus impairing growth and production.61
Coccidiosis: It is the most common diseases of poultry resulting in great economic losses in developing country. It is the most common disease of chickens which caused by protozoal parasites. Eimeria species include Eimeria tenella, E. brunetti, E. mitis, E. acervulina, E. necatrix, E. maxima, E. mivati. While the parasite belonging to species Emeria mivati was the least abundant, the most parasites belonging to protozoa indicated the highest predominance through the wet period than the waterless period, telling us that warm environmental conditions and lower humidity favor the development of this parasite. Clinical signs of parasitism are lack of development, feed conversion, poor growth, low egg production, and even death in severe infections. Moreover, the parasites can make the herd less resistant to disease and exacerbate existing disease in the herd.62,63
Ectoparasites: The most common ectoparasites that cause disaster to poultry health and production range from fleas, ticks, mites, and lice. They may cause clinical problems and transmit a number of infectious diseases and can act as transporters or intermediate hosts of a range of helminth parasites.64 Ticks and mites act as vectors of poultry diseases such as Newcastle disease, chlamydia, and pasteurellosis.65,66
Poultry Health Management
Health management is a preventative approach that addresses the complete animal and all impacts on its health, including social, psychological, and environmental variables. Poultry housing should be weather-proof to protect the birds from the elements (cold, rain, wind, and the hot sun) as well as offer warmth, particularly during brooding.67 Cleaning and disinfection entail the physical and chemical removal of contaminated debris (typically with detergent and water) as well as the decrease or elimination of pathogenic organisms in or on materials such that they no longer pose a health risk. Evaluating the biosecurity of ongoing operations is important in developing effective programs to prevent the introduction of disease into a complex or to limit subsequent dissemination among farms. Vaccination is a measure that may be applied wherever a high risk of introduction and further spread of a contagious poultry disease has been identified.67,68
Proper feeding and watering
The feeding management of poultry in Africa is not appropriately measured in the traditional ways. Generally no supplements are provided except that sometimes household waste is fed to the birds. Similarly, in Ethiopia, chicken production is characterized by a free-range system with some supplementary feeds such as frushika, maize, sorghum, and food leftover, and the major feed sources are thought to be insect worms, seed, and plant materials.69 However, the availability of the supplementary feeds was reported during the dry season (November to March) following the grain harvest while the grains and grain by-products were in short supply, leading to feed scarcity during the rainy season.70 Modern broiler, breeder, and egg-production flocks require balanced diets consisting of essential nutrients to achieve optimal reproductive efficiency, feed conversion, live ability, and immune response. Starter rations are high in protein an expensive feed ingredient. However, grower and finisher rations can be lower in protein since older birds require less.69
A lack of water will reduce feed intake, seriously retarding growth and impairing egg production. This is particularly true in hot climates, where deprivation can rapidly lead to death. Birds need a lot more water at high temperatures than at low temperatures, and a lack of water quickly leads to death by overheating.71,72
Poultry housing
There are specific factors that jeopardize and increase the spread of the parasite, including inadequate biosecurity protocols and poor hygiene of both personnel and equipment. Sanitization plays a major role in reducing the dissemination of the parasite as the most frequent mode of transmission of oocysts is through mechanical vectors such as movement of personnel or equipment between farms and the presence of rodents and insects such as flies and beetles.73,74
Poultry housing should be weather-proof to provide protection from the elements (cold, rain, wind, and hot sun) and predators and provide warmth.75 Chickens begin panting at 29.4°C to help dissipate heat and drink more to avoid dehydration due to they have no sweat glands. Proper ventilation will also help regulate the house temperature. Each house should have a thermometer to display the current temperature as well as the high and low temperatures in a daily period, and producers should pay attention to weather forecasts.76
Ventilation brings fresh air into a poultry house and removes heat, moisture, and gases. Natural ventilation makes use of the movement of air (warm air rises and cold air falls) and wind currents. A roof at least six feet tall will allow a sufficient height differential for cool air to enter through low air inlets and warm air to escape through high vents.77 There is less control in natural ventilation than in mechanical. During warm months, the purpose is to remove heat and control the temperature in the house, and, therefore, large amounts of air are moved. During cold months, the ventilation system must remove moisture and gases, especially ammonia, while conserving heat. This is tricky because producers tend to keep houses closed up tight to conserve heat.78 It is done by controlling air inlets and is possible because warm air holds more moisture than cold air does. As a result, during cold weather, producers can bring small amounts of high moisture air into the house, allow the fresh air to heat to room temperature, and when this air leaves, it takes moisture out of the house.75 Poultry are very sensitive to light. Light not only allows them to be active and find their food, but it also stimulates their brains for seasonal reproduction.79 Chickens need a dark period for good health. They only produce melatonin an important hormone in immune function during dark periods.80 Welfare programs usually require at least four to six hours of darkness daily. Fast-growing broilers can be especially helpful in the first weeks of life to slow growth, build a frame, and reduce leg disorders. Growers need 15 hours of light per day; layers need 17 hours of light per day.78
Sanitation
The goal of sanitation is to decrease or eliminate bacteria populations that are harmful to flock health. Cleaning and disinfection entail the physical and chemical removal of contaminated debris as well as elimination of pathogenic organisms in or on materials such that they no longer pose a health risk.81 Wet cleaning is done in a systematic manner, moving carefully from one short side of the home to the other, from the rear to the front of the building and from the top downwards. For building disinfection, a 4 percent formalin with propylene glycol is recommended. Propylene glycol is required for formaldehyde to permeate pores, cracks, and crevices between metal plates that are riveted or welded together. With the introduction of foaming techniques, the contact time of disinfectants has extended by many times. Because foam takes a long time to dry, the disinfectant's antibacterial action is substantially amplified. Following a disease outbreak, hygiene and disinfection must be high on the priority list for infection control.68,82
Biosecurity
Bio-security is a set of techniques that prevent disease causing organisms from spreading. In order to establish effective programs to avoid the entrance of illness and limit its spread among farms.83 To maximize benefits, an effective bio-security program necessitates an understanding of epidemiology and economic principles, as well as teamwork. Bio-security programs necessitate a systematic approach that includes the following steps: program planning and evaluation; locating resources and personnel training; implementing, which includes the construction of facilities; and controlling, which includes the review of results and analytical procedures.84 The bio-security of the village poultry production system is very poor, as scavenging birds live together with people and other species of livestock. Poultry movement and droppings are very difficult to control, and chickens freely roam in the household compound. There is no practice of isolating sick birds from the household flocks, and dead birds are left for either domestic or wild predators.85,86
Chickens and eggs are sold on open markets along with other food items. The current live bird marketing system represents a significant and potential hazard to both buyers and sellers, yet implementation of biosecurity and hygienic practices in such a system is generally difficult.79 The primary level is the foundation for all disease prevention efforts. The arrangement of a complex or operation in a specified region to separate different types of poultry, limit bio-density, and avoid contact with free-living birds is referred to as conceptual bio-security.87
Biosecurity approach can be categorized as structural and operational biosecurity. Farm layout, erection of fences, construction of drainage and decontamination equipment, change rooms, and the exclusion of rats and wild birds are all part of structural biosecurity.88 Routine management methods aimed at preventing the entry and spread of illness within an organization are referred to as operational biosecurity. These activities can be changed on the fly in the event of a disease outbreak. Effective operational bio-security requires constant assessment of protocols, participation from all levels of management and labor, and appropriate monitoring of flock health and immunity.67
Vaccination
Vaccination has been used successfully over the world since the 1940s and is regarded the most effective method of managing diseases. When a disease strikes, the quality of the vaccine is sometimes blamed. However, other problems, such as a lack of a cold chain, are frequently to blame. Identifying the reasons and resolving the problem frequently necessitates a thorough investigation.80 Vaccination is a measure that may be applied wherever a high risk of introduction and further spread of a contagious poultry disease has been identified. The scientific basis for the use of this strategy is the generation of a level of protective immunity in the target population that can be boosted in case of immediate risk or evidence of introduction of a field virus. The use of vaccination in the absence of any outbreak of disease, together with the application of effective bio-security measures, could maximize poultry protection whenever a risk of exposure exists. Vaccination is generally carried out for the prevention of poultry diseases that have a clear impact on the industry.83 The common vaccination for poultry disease is summarized as Table 1.
|
Poultry |
Vaccine |
Type |
Administration route |
|
Layers |
Marek’s disease |
Live |
Subcutaneous injection |
|
Newcastle |
Live |
Drinking water (>14 days), revaccination (>4 weeks) |
|
|
Infectious bursal disease |
Live |
Drinking water (>7 days) |
|
|
Encephalomyelitis |
Live |
Wing web (>8 weeks and 4 weeks before start of lay) |
|
|
Fowl pox |
Modified live |
Wing web (>8 weeks and 4 weeks before start of lay) |
|
|
Laryngotracheitis |
Modified live |
Intraocular (>4 weeks) |
|
|
Mycoplasma gallisepticum |
Live |
Intraocular or spray (>9 weeks) |
|
|
Broiler |
Marek’s disease |
Live |
Subcutaneous injection |
|
Newcastle disease |
Live |
Drinking water (14-21 days, spray revaccination>4 days |
|
|
Infectious bronchitis |
Live |
Drinking water (14-21 days, spray revaccination>4 days |
|
|
Infectious bursal disease |
Live |
Drinking water (7 days) |
|
|
Broiler breeder |
Marek’s disease |
Live |
Subcutaneous injection |
|
Newcastle disease |
Live, Lasota staris |
Drinking water (14-21 days, spray revaccination>4 days |
|
|
Infectious bursal disease |
Live |
Drinking water (<28 days), spray (>8 weeks) |
|
|
Encephalomyelitis |
Live |
Wing web (>8 weeks) |
|
|
Fowl pox |
Modified live |
Wing web (>8 weeks) |
|
|
Fowl cholera |
Modified live |
Wing web (>10 weeks), subcutaneous injection |
|
|
Laryngotracheitis |
Live attenuated |
intraocular (>4 wks.) |
Table 1 The common Vaccination for poultry disease89
Disease of Swine
Disease among pigs’ lead to reduce productivity by reducing feed conversion efficiency, slowing growth rate and increasing mortalities.90 Respiratory problems, limb problems (lameness, hoof injuries, and abscesses), reproductive disorders (abortion), gastrointestinal problems, claw and skin problems, parasite infections, and piglet mortality are the most common health issues in pig husbandry. Skin trauma or sow crushing of piglets, as well as diarrhea syndromes in suckling and weaning piglets, increase piglet mortality. The quality and hygiene of the outside space, interior cages, and wallowing holes are all risk factors for diarrhea in weaned piglets.91
The most common clinical sign of disease in pigs is behavioral changes such as abnormal lying, loss of appetite, depression and disorientation, as in encephalomyelitis and Aujesxky’s disease. Other symptoms include lameness, labored breathing, excessive salivation, vaginal discharge, coughing, swollen joints, scouring, diarrhea, and the presence of external parasites.92 Diarrhea is the most common recorded disease among pig farms, followed by skin, worm infection and anorexia. The diarrhetic pigs are inactive and vulnerable to severe secondary infections and death. Bacterial disease is the most commonly occurring disease in swine production, which causes respiratory, enteric, and septicemic infections. The most common bacterial disease in swine is detailed in Table 2. The most common respiratory problems with signs of pneumonia are usually caused by Mycoplasma Hyopneumoniae, Actinobacillus pleuropneumoniae and Porcine Reproductive and Respiratory Syndrome Virus.91 There are several risk factors for the occurrence of disease in swine production. The factors may include genetic factors, host factors, environmental factors, and vectors. The important risk factor for disease in swine production is direct contact with rodents. Rodents are the vectors that carry several pathogens, even some of them zoonotic, such as Trichinella spp., Toxoplasma gondii, Salmonella spp., Campylobacter and Leprospira.93–95
|
Forms of disease |
Bacterium |
Disease |
Susceptible age of pig |
|
Enteric |
Eschercial coli |
Neonatal scours |
1 day |
|
Piglet scours |
7-14 days |
||
|
Post weaning diarrhea |
5-7 days after weaning |
||
|
Brachyspita hyodysentiae |
Swine dysentery |
Growers and finishes, -2 weeks, all age in primary breakdown |
|
|
Salmonella |
Typhimurium: diarrhea, septicemia, death |
Growers’ pigs -16 weeks |
|
|
derby: diarrhea |
Growers’ pigs |
||
|
choleraesuis: Septicemia, diarrhea, death |
Finishing pigs 12-1 weeks |
||
|
Clostridium perfringens |
Type A-diarrhea |
10-21 days,weaned pigs |
|
|
Type c- necrosis enteritis |
1-7 days |
||
|
Clostridium difficile |
Diarrheal thrift |
3-7 days |
|
|
Septicemic |
Streptococcus suis |
Meningitis, endocarditis, arthritis and peritonitis |
2-10 weeks |
|
Haemophilus parasuis |
Glasser’s disease |
2-10 weeks |
|
|
; arthritis, pericarditis, peritonitis |
|||
|
E.coli |
Bacteremia,arthritis,navel ifectins, cystitis,nephritis |
Post weaning sows |
|
|
Mycolasma hyosynoviae |
Mycoplasma aerthritis |
16 weeks |
|
|
Staphylococcus aureus |
Bacteremia, arthritis, osteomyelitis,mastitis,metritis |
All ages |
|
|
Sthyloccoccu hyicus |
Exudative epidermis |
Pre and post weaning pigs |
|
|
Erysipelothrix rhusiopathies |
Erysipelas (dematitits,diomond like scar on skin,arthritis and endocarditis |
Growers, finishers and sows |
|
|
Respiratory |
Pasteurella multocida |
Atrophic rhinitis |
1-8 weeks |
|
Bordetella bronchiseptica |
Atrophic rhinitis |
Nasal distortion lasts for life |
|
|
Mycoplasma hyopneumoniae |
Enzootic peumonia |
Growers and finisher pig |
|
|
Actinobacillus pleuropneumonia |
|||
Table 2 The common bacterial disease of swine101
Important risk factors for leg problems in sows are considered to be genetic factors affecting leg strength, diseases in legs and hooves, ground condition in outdoor areas and management in the mating area. Poor mating management regarding oestrus and pregnancy testing, synchronization of oestrus in sow batches, and poor body condition are regarded as important risk factors for reproductive problems in the herd.96 The other pig disease is endoparasite and ectoparasite infestation, which is detailed in Table 4. Endoparasites and ectoparasites is considered as the major constraints for welfare and health as well as economic loss in swine production, especially during post weaning period. More frequently, parasites in pig farms are Sarcoptes scabies, Trichuris suis, and Ascaris suum. Ascaris suum causes growth retardation and milk spot lesions in the live.97–99 In the swine industry, chronic viral infections cause long-term health risks, resulting in significant financial losses around the world. Virus infections such as porcine reproductive and respiratory syndrome virus, swine influenza virus, porcine epidemic diarrhea virus, porcine circumcovirus, foot and mouth disease virus, and others have a high economic cost because they cause severe morbidity, mortality, loss of production, trade restrictions, and investments in control and prevention practice.100 and also detailed in Table 3.
|
Disease |
Virus |
Clinical signs |
|
Porcine reproductive and respiratory sysndrome |
PRRS virus, single stranded +RNA |
Fever,anorexia,mild to severe reproductive problem, abortion, reproductive failures |
|
Foot and mouth disease |
FMD virus single strand + RNA |
Fever inappetence,vesicular lesions on extremities |
|
Swine influenza |
Influenza A virus,single strande - RNA |
Fevr,anorexia,loss of weight gain,respiratory problems |
|
Porcine epidemic diarrhea |
PED virus,single strand +RNA |
Severe diarrhea,vomiting and dehyration |
|
Classic swine fever/hog cholera |
CSF,Single strand +RNA |
Fever,anorexia,erythema,respiratory signs,neurological signs, reproductive failuries ,death |
|
Porcine circovirus associated disease |
Porcine circovirus 2, single strand DNA |
Poor weight gain,respiratory problems, dermatitis, enteritis, nephropathy, reproductive failures |
|
Porcine parvovirus |
Ungulate parvovirus 1, single strand DNA |
Stillbirth, mummification, embryonic death, infertilities |
|
Pseudorabies,Aujezly’s disease |
Suid herpesvirus 1, double strand DNA |
Nervous disorders,respiratory problems,weight loss |
|
African swine fever |
ASF virus, double strand DNA |
Fever, anorexia,erythema,respiratory signs,reproductive failures,death |
Table 3 The common viral disease of Swine100
|
Parasite |
Infection site |
Clinical sign |
|
|
Trichuris suis (Whip worm) |
Large intestine |
Diarrhea and dehydration |
|
|
Trichinella spiralis |
Muscle |
Uncommon, exist at meat inspection |
|
|
Strongloides ransomi (Threadworm) |
Small intestine |
Diarrhea |
|
|
Hyostronglus rubidius(Red worm) |
Stomach |
Emaciation, anemia |
|
|
Oesophagostomum(Nodular worm) |
Large intestine |
Reduced performance |
|
|
Metastronglus(Lung worm) |
Lungs |
Coughing, pneumonia |
|
|
Ascaris suis(Large roundworm) |
Liver damage, reduced performance |
||
|
Stephanurus dentatus |
Kidney |
Wasting, blood in urine |
|
|
Coccdia |
Small intestine |
10-day old scour |
|
|
Toxoplasma |
muscle |
Abortion |
|
|
Ticks |
Skin |
No Lesions, feed on blood |
|
|
Flies (stable, blow,black |
Skin,body of pig |
Skin lesions, small papules |
|
|
,horse,screwworm,house) |
|||
|
Sarcoptic mange |
Skin |
Irritation, rash, thickened skin |
|
|
Lice |
skin |
Exist on ear, no lesions |
|
Table 4 The common parasites of swine102
Swine Health Management
The most common stressful practices that cause immunosuppression and trigger disease onset include early weaning, transportation, inadequate temperatures or air draught, scarcity of feed, and lack of health management.103 Disease and injuries are the key elements during the monitoring of the welfare and health management of swine. Health management of swine is an important issue for optimizing productivity.104
Health management practices contribute to animal well-being by providing an approach for effective treatment, rapid diagnosis, and disease prevention. Herd health management practices include vaccination; observation of all animals' clinical signs; record keeping; detection and treatment of injury; sanitization; disease; pest control; and animal handlers. Prevention of disease is preferable to treatment of disease for animal welfare and is more cost effective for the producer in swine production.105,106 To generalize, the herd health management of swine has its own framework status that aims to identify the level of engagement and willingness to perform any intervention of management, productivity, assessment of health and welfare. And also practicing reproductive management, health management, and biosecurity. Thus, the health management of swine farms has its own framework which includes the assessments and practices.107 The frame work of herd health management is described in Figure 1.
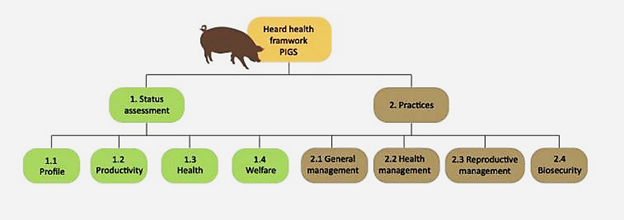
Figure 1 Framework of swine health management.107
Antimicrobial therapy used for treatment
Pathogens that cause swine diseases include bacteria, parasites, and viruses. The antimicrobials are employed to treat bacterial infections and also anthelmintic for the purpose of deworming. However, viral diseases have no cure but prevention can be applied via vaccination.108 The important principles and regulations for animal treatment include: the treatment requirements that animals must immediately get treatment; complementary medicine must be applied (to support the defense mechanism of organisms without leaving chemical residues in dung and food (homeopathic and phototherapeutic products); and the doubling of the legal withdrawal period for chemical drugs in pig farming to improve the desired consumer protection. In addition to this, prebiotics, probiotics, and phytogenic are employed as potential alternatives to antibiotics in swine diets.109–111. Improved digestion, gastrointestinal immunity stimulation, and higher resistance to gastrointestinal infectious illnesses are among claimed benefits of probiotics.112,113 Probiotics containing Bacillus cereus, Bacillus licheniformis, or Bacillus subtilis spores, for example, have been shown to improve the health and performance of sows, as well as weaning, growing, and finishing pigs, as well as the incidence and severity of Post Weaning Diarrhea Syndrome (PWDS), which is caused primarily by enterotoxigenic E. coli - ETEC strains.114 Phytogenic have significant antimicrobial activity against bacteria (especially Gram-negative bacterial species, like Salmonella spp. and E. coli) yeasts, moulds and parasites (like Ascaris suum).115–118 These products potentially provide also antioxidative effects, enhance palatability, improve gut functions, or promote growth.119–121
Genetic improvement
Pigs that have undergone genetic enhancements have become more resistant and hardier. It's the first step toward inducing resistance to major disease pathogens.122 The swine genome has enabled for genetic selection of specific features, according to the findings. For decades, this process has been going on, with breeders looking for qualities that produce the best carcasses, larger litters, and other phenotypic attributes. Understanding genetic disease resistance has enabled for the selection of pigs that are resistant to specific diseases in recent years. Pigs that were resistant to E. coli's K88 antigen due to a genetic deficiency in a receptor were found. Recently, gene editing has been used to develop pigs that are genetically resistant to PRRSV.123,124 As a result, genetically modified pigs may develop resistance to devastating infectious diseases. So, to conclude, genetic improvement has a great role in the management of swine health.125
Biosecurity
Health programs are constantly providing the best information on biosecurity at the individual farm, production system, and industry level. Regular personnel training also ensures that all in the industry are meeting the standard for animal health and sustainability.126 Biosecurity is the combination of practices that are designed to reduce the introduction and spread of disease. Biosecurity is an important aspect of preventing the transmission of diseases, thus improving health and reducing the need for antimicrobials (AMs). Biosecurity best practices include isolating new swine from susceptible herds, quarantines, using disinfected instruments, and practicing good hygiene. External biosecurity aims to keep transmissible pathogens out of the herd, while internal biosecurity prevents the spread of disease, mainly from older to younger animals within the herd.127 Porcine Respiratory and Reproductive Syndrome Virus (PRRSV) is the most economically important disease in the swine industry. As such, many of the biosecurity protocols focus on the exclusion, management, and containment of PRRSV. Other diseases may cause slight deviations in protocols specific to farms.128
Feeding and watering
The main factors for the enhancement of swine production quality are feeding and watering. Pigs need access to daily feed that is palatable, fresh and free of gross contamination. Sound feeding practices that provide for adequate nutrient needs are integral to the health and wellbeing of pigs in all stages of production.129
The major feed sources of swine are grass, brewer’s residue, corn, and soybeans, feed additives (vitamins, minerals, protein sources, and energy sources may come from various suppliers), rotten fruits, food waste (garbage), waste containing meat, crop residues, and ruminal contents from the local abattoirs.130–131 However, the feed that is brought to farms on a regular basis can serve as a source of disease agents. Not only that, but the process of delivering feed to the farm poses a disease risk because the trunk brought from the feed mill can spread potential pathogenic disease to the subsequent farm. The farms that utilize a commercial feed mill have the highest risk of disease compared to those that have the opportunity to mill their feed on the premise. Recent experiences with disease outbreaks, namely the Porcine Epidemic Diarrhea Virus, have raised concern with the source of commodities brought into the mill.132 Therefore, using qualified feed and supplying swine may increase the productivity of swine and disease-free farms.
Pure water is another essential component that must be provided to confined pigs. Drinking water delivery techniques might have a negative impact on one's health. Although trough delivering systems are generally affordable, the flow of water through multiple pens or groups of animals can transfer disease to others further downstream. The pulling that delivers the alter to the pigs could become an infection source. As a result, clean waterlines, whether mechanical or chemical, can minimize water contamination and regulate infectious disease spread throughout swine farms.133–135 Water is also used for sanitation, which poses a risk of disease outbreaks and a potential disease control area. The excrement is collected in an effluent pit beneath the slatted floors of many modern swine barns. The pits are flushed to lagoons on a regular basis. At the time of flushing the pit, the waste gets agitated potentially releasing pathogens or noxious gases to make animals in the barn ill. The use of recycled water for flushing pits may also reintroduce pathogens into the farm.136
Swine housing
Swine housing is used to keep pigs safe and to improve their living conditions. Pigs in modern swine farming systems are kept comfortable and stress-free while simultaneously providing the best possible environment for their immune systems. Temperature, ventilation, feed delivery, water distribution, and cleanliness are all frequently managed automatically. Animals can be segregated from one another using confinement devices. To improve biosecurity, each swine raising site can be segregated, as previously discussed. Other animals can also be kept away from pigs. Pests such as rats and birds that can carry disease to and from swine can be kept out of barns. To increase the distance and reduce the possibility of disease spread, barns should be at least one mile apart. To achieve the optimal temperature, humidity, and air exchanges, temperature and ventilation are coordinated. Temperature and ventilation are coordinated to provide the ideal temperature, humidity, and air exchange. Heaters and evaporative coolers provide temperature variations.137 Direction of the airflow through creation or prevention of drafts helps to alleviate heat stress in hot months and prevents young people from becoming chilled and susceptible to commensal pathogens. Attention to the ventilation rates is important in these systems to rid them of noxious gases that can irritate the respiratory system and increase the vulnerability to disease. The addition of air filtering systems to intake vents creates a layer of protection against airborne diseases. Positive pressure ventilation in barns drives air out of barns, making it a critical part of biosecurity.138,139
Animal movement and transportation
The introduction of new breeding animals into the herd poses the biggest biosecurity risk. Replacement gilts should be quarantined for at least 30 days on the farm. Animals are tested throughout the isolation period to determine their disease status and provide assurance before being reintroduced to the herd. There should be an acclimatization time for fresh arrivals to be introduced to elder sows during the seclusion period. Older, cull sows, unwell animals, or feedback from farm materials can expose naive gilts to farm-specific disease strains that immunization programs may not be able to prevent. Any disease that can be transferred through reproductive fluids is a risk with sperm sent to the farm. Boar studs are kept as one of the most secure areas in a production system to protect the sow farms that receive semen from the farm.128,132
In the boar stud, disease has the ability to quickly spread to thousands of animals in a short period of time. Independent farms may receive semen from various sources, increasing the danger of disease introduction, whereas sow farms with integrated systems receive semen from a single boar stud source, reducing disease risk. The shipping materials used to bundle sperm for delivery could also act as a vector for disease transmission. To avoid the spread of infections on the farm, disinfection procedures should be implemented. Animals leaving the grounds can even endanger the farm by introducing vehicles that have gone to the destination premise and returned filthy. Those animals pose a threat to the receiving premises, necessitating possible movement limitations or communication between farms to prevent disease spread. Breaking the disease cycle as younger, more susceptible animals are brought into the herd requires timely removal of culls and animals as they mature to the next stage of production. Swine farming has become increasingly specialized as a result of the realization that other animals can carry disease to pigs, among other reasons. Rodents and birds can be both hosts and predators.132
In the management of disease, people, animals, and supplies going to and from farms pose a substantial danger. Each of the several sorts of vehicles that visit a farm poses a distinct amount of risk. It is recommended that personnel cars park on the farm's boundary. Similarly, placing feed bins near the farm's boundary permits feed delivery trucks to stay outside. Utility meters are placed in such a way that meter readers are kept at a safe distance from animals.128 One of the largest hazards of disease spread is when animals are transported in trailers. The farm's load-out areas are the most dangerous. Truck drivers should remain in their cars, with agricultural employees handling loading and unloading. Truck drivers should stay in their vehicles with loading and unloading managed by farm staff. All trailers should be thoroughly cleaned and disinfected between loads. Truck wash stations may provide a suitable location for cleaning. Public washes run the risk of introducing diseases from other farms, so private washes may be prudent in areas with a number of farms under the same management. Some pathogens have brought to light the need to have a heat drying stage in the cleaning and disinfection process to fully de-activate pathogens. Load-out areas should be cleaned and disinfected as they are the transition areas to the farm. Transport of dead animals to rendering poses another concern, as the trucks used for that transport are often exposed to unknown pathogens. Animals that die or are euthanized on the farm should be removed from the barns immediately and placed in a protected location until they can be removed from the farm. Vehicles removing carcasses should stay as far from live animals as reasonably possible, keeping in mind visibility of roads, neighbors, and others. It is paramount that farm employees verify the cleanliness of vehicles entering the farm boundaries and deny entry to those that may pose a disease risk.132
Personnel
Employees in high-health farms have access to shower facilities. Clean/dirty lines demarcate where employees can be supplied the clothing they're wearing. On one side of the shower, street clothes are abandoned, hair and bodies are washed and rinsed, noses are blown, and farm-specific attire is put on. When handling animals, appropriate personal protective equipment (such as gloves) should be worn to protect employee health and prevent the transfer of infections from one pig to the next. A different pair of boots should be worn if work involves loading shipments, removing dead animals, or caring for gilts in their place. High-risk activities, such as these, are ideally delayed until the end of the day as returning to the inside of the barn requires another shower and change of clothes. Additional time between performing these duties and interacting with the other pigs on the farm reduces the risk of spreading disease. Some farms recognize swine ownership by their employees as a risk of disease extending between herds. Employees may be restricted from travel to areas with other livestock, specifically swine. Forty-eight hours has historically been suggested as a necessary downtime for exposure to other swine domestically.132
Bio management (vaccination and treatment)
Specific immunizations that are largely unsuccessful in other herds benefit some systems. Movement controls, disease surveillance, immunization, and other biosecurity measures are used to control complicated diseases, such as PRRS. Given an understanding of the condition, treatment methods are the result of the connection between a veterinarian and a customer (herd) of instance antibiotics used. These allow for farm employees to recognize and treat common diseases at a veterinarian’s direction. Producers are encouraged to recognize pigs that do not respond to treatment in a timely manner so that alternatives can be sought for the betterment of pig welfare and herd health. The training the personnel undergo, biosecurity protocols, vaccination programs, and a strong relationship with the veterinarian serve to appropriately use antibiotics and improve pig health.132,140 Vaccinations against porcine parvovirus, Escherichia coli, Mycoplasma hyopneumoniae, PRRSV, and Aujeszky have to be applied in regions with a large number of conventional swine farms. Preventive vaccination programs are important, especially in regions where there are swine farms.97
Swine and poultry production have a great role in the development of the economy in developing countries. The production of these animals may provide high-quality protein meat with a balanced cost. However, swine and poultry production can be influenced and suffer from different factors, including infectious and non-infectious diseases. The viral diseases are the most fatal and highly mortal diseases among poultry and swine. The economic losses from swine and poultry production result from the harmful effects of disease that cause decreased growth rates, decreased meat and egg production, and, indirectly, the export income is diminished from the fatal diseases. This entire economic failure can be solved with the application of health management on swine and poultry farms. Animal health management begins with management of the farm, from nutrition to biosecurity. Feeding and housing management play a crucial role in the control of diseases. Biosecurity is also the most important in the control and prevention of the spread of disease among farms. Generally, disease has a great risk to the health of poultry and swine animals that causes a decrement in their production. Good quality meat and eggs can be produced with good health management. Therefore, the standardized poultry farm should be founded in Ethiopia, which would create job, the health of swine and poultry should be followed up routinely, the common animal disease of poultry and swine should be studied well, the biosecurity should be measured in the farm of poultry and the government should address and support youth to produce swine and poultry farms.
The reviewers and editors deserve our gratitude for their insightful work, insightful comments, and helpful corrections to the manuscript, all of which helped us make it better. Additionally, the authors have a great deal of gratitude to the researcher because they used references to cite their work in this review. The authors would also like to express their gratitude to the International Journal of Avian and Wildlife Biology for accepting and retrieving their manuscript. Last but not least, the authors would like to acknowledge and appreciate their colleagues' appreciation and encouragement to conduct scientific research.
The authors declare that there are no conflicts of interest.

©2023 Serbessa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.