International Journal of
eISSN: 2574-9862


Research Article Volume 8 Issue 3
1Veterinary Hospital, Dubai Safari Park Section, Dubai Municipality Dubai, United Arab Emirates
2Operations Unit, Dubai Safari Park Section, Dubai Municipality Dubai, United Arab Emirates
Correspondence: Murad B Mustafa, Veterinary Hospital, Dubai Safari Park Section, Dubai Municipality Dubai, United Arab Emirates
Received: September 01, 2024 | Published: September 12, 2024
Citation: Mustafa MB, Herawati NM, Talukdar A, et al. Individual immune response to rabies vaccination in an African Wild Dog (Lycaon pictus) in Dubai Safari Park. Int J Avian & Wildlife Biol. 2024;8(3):92-96. DOI: 10.15406/ijawb.2024.08.00220
This research examines the efficacy of a monovalent rabies vaccine in immune-compromised African wild dogs (Lycaon pictus) and the impact of stress reduction on vaccine response. Stress, due to transportation and acclimatization to a new environment, adversely affects the immune system and can reduce vaccine efficacy. Our findings demonstrate that while a combination of vaccines resulted in a lower antibody titer, the monovalent rabies vaccine significantly improved antibody levels after stressors were removed. Despite this improvement in immune response, reintegration of the treated individual into the pack remains problematic due to challenges associated with the separation and subsequent social dynamics post-treatment. This underscores the importance of managing stress and providing individual care to enhance vaccine efficacy and ensure successful social reintegration.
Keywords: African wild dog, monovalent rabies vaccine, immune response, rabies titer, welfare
Dubai Safari Park (DSP) is a leading animal theme park that boasts a diverse collection of over 3,000 animals. Committed to conservation education, the park strives to raise awareness and promote the protection of wildlife. It also plays a crucial role in the responsible management of captive animals through its animal exchange program, which facilitates finding homes for surplus animals bred in captivity, both within its own facilities and in other safari parks. Additionally, the park receives various species through donations, further enhancing its conservation and educational initiatives. At the end of 2023, Dubai Safari Park received a donation of four African wild dogs for its future breeding program, a significant addition that supports the park’s ongoing conservation and educational goals. It is estimated that there are around 6,600 adults (including 1,400 mature individuals) living in 39 subpopulations, all threatened by habitat fragmentation, human persecution and outbreaks of disease. As the largest subpopulation probably consists of fewer than 250 individuals, the African wild dog has been listed as endangered on the IUCN Red List since 1990.1
Kennedy et al,2 observed that young animals, less than 1-year of age, generated a lower antibody response to rabies vaccination than adults. Considerably higher failure rates were also observed for different vaccines tested. Regression analysis revealed that two vaccines performed equally well, and significantly better than the others tested. The variation in antibody response relating to length of interval of sampling following vaccination is not unexpected and presumably relates to the response kinetics for primary vaccination. Minke;3 suggested that the choice of the vaccine and the timing of blood tests are critical factors in achieving successful serological test results after rabies vaccination.
The aim of this study was to evaluate the effectiveness of different vaccine products in eliciting immune responses in wild dogs, focusing on antibody titer levels and the impact of factors such as stress and health status on vaccine efficacy.
Object
This African wild dog (Lycaon pictus) was born on May 13, 2021, at the Réserve Africaine de Sigean in Aude, in the south of France. All four wild dogs for the first time on June 2, 2023, received Rabigen Mono and Nobivac L4 vaccinations at the same facility. Upon arrival at the quarantine facility in Dubai Safari Park, a booster dose of Biocan DHPPi + LR was administered to four of these wild dogs, on December 17, 2023, during the first week of quarantine (Figure 1) (Table 1).
|
African wild dog |
Body weight |
|
Ukunda (250 228 500 015 771) |
24.04 |
|
Ushabti (250 228 500 015 895) |
23.3 |
|
Utopia (250 228 500 015 604) |
20.86 |
|
Uele (250 228 500 015 770) |
18.05 |
Table 1 Animal list
Sample collection
All wild dogs were fasted for one day prior to blood sample collection. Before examination, the wild dogs were sedated within their enclosures using anesthetic darts containing dogs were administered ketamine (4.5 mg kg−1), medetomidine (0.04 mg kg−1) intramuscularly. At the end of the procedure, medetomidine was antagonised with atipamezole. anesthetized with ketamine or propofol, medetomidine is a satisfactory sedative-analgesic premedicant.4 The animals were then weighed, and 5 ml of blood was collected aseptically from their femoral vein using a 21G x 1.5” needle. The samples were transferred to EDTA and clot activator tubes. The supernatant (serum) extracted from the clot activator tubes was used for biochemical analysis and to titer Ab rabies test, while the EDTA samples were used for hematological analysis.
In this research there are two locations of withdrawing blood, those are in femoral vein and cephalic vein. The cephalic vein is commonly used for intravenous infusions but may not be ideal for "arterialized" venous blood sampling. The saphenous vein in the hind leg is preferred for this purpose. "Arterialized" venous blood sampling involves obtaining blood with characteristics similar to arterial blood, such as higher oxygen content and pressure. (Figure 2 & 3)5 The advantage to femoral vein bleed is that a larger volume is more easily collected than from the saphenous vein. Arterial access, used for monitoring blood pressure and sampling, is commonly done via the dorsal pedal artery, but the radial, brachial, femoral, and auricular arteries can also be used in specific cases.7 All blood products should be clearly labeled with the date, time of collection, donor identification, and blood type. Aseptic protocol must be used when preparing components or handling whole-blood products.8
Hematology and biochemical analysis
Hematology tests assess various components of blood, including red blood cells, white blood cells, and platelets. They help diagnose conditions such as anemia, infections, and blood disorders.9 Biochemistry tests measure various chemical substances in the blood, including electrolytes, proteins, enzymes, and metabolites. It helps to assess organ function (liver, kidneys, pancreas) and monitor metabolic health.10
Analysis
Analysis method is a Rabies Neutralizing Antibody Titration Test by Fluorescent Antibody Virus Neutralization (FAVN) method. Then comparison of the hematology result with improvement of animal behavior (Table 2 & 3), (Chart 1).
|
Date |
17-Dec-23 |
15-Feb-24 |
15-May-24 |
|||||
|
Component |
Unit |
Reference Interval |
Utopia |
Ukunda |
Ushabti |
Uele |
Uele |
Uele |
|
WBC |
109/L |
7.574-14.606 |
7.84 |
10.9 |
7.46 |
8.71 |
6.12 |
6.86 |
|
Abs. Lymphocyte |
109/L |
0.574-2.94 |
1.05 |
1.07 |
0.85 |
1.35 |
0.61 |
0.42 |
|
Abs. Monocyte |
109/L |
0.145-0.835 |
0.24 |
0.3 |
0.52 |
0.4 |
0.3 |
0.5 |
|
Abs. Neutrophil |
109/L |
5.489-11.173 |
6.14 |
9.17 |
5.78 |
6.24 |
5.13 |
5.74 |
|
Abs. Eosinophil |
109/L |
0.143-1.141 |
0.29 |
0.23 |
0.23 |
0.58 |
0.05 |
0.14 |
|
Abs. Basophil |
109/L |
0.006-0.224 |
0.12 |
0.14 |
0.08 |
0.13 |
0.02 |
0.05 |
|
Lymphocyte % |
% |
- |
13.4 |
9.8 |
11.4 |
15.6 |
10 |
6.2 |
|
Monocyte % |
% |
- |
3 |
2.7 |
7 |
4.5 |
5 |
7.2 |
|
Neutrophil % |
% |
- |
79.4 |
8.4 |
77.5 |
71.7 |
83.8 |
83.7 |
|
Eosinophil % |
% |
- |
3.7 |
2.1 |
3 |
6.7 |
0.9 |
2.1 |
|
Basophil % |
% |
- |
1.5 |
1.3 |
1.1 |
1.5 |
0.3 |
0.8 |
|
RBC |
1012/L |
6.86-9.28 |
10.42 |
8.72 |
9.38 |
6.76 |
9.47 |
8.39 |
|
HGB |
g/dl |
13.5-17.3 |
18.2 |
15.8 |
16.5 |
16.2 |
16.4 |
14.8 |
|
HCT |
% |
38.4-50.2 |
56.11 |
47.54 |
50.13 |
51.85 |
49.55 |
44.78 |
|
MCV |
fl |
51.3-59.7 |
54 |
55 |
53 |
53 |
52.32 |
53 |
|
MCH |
pg |
17.4-20.8 |
17.5 |
18.1 |
17.6 |
16.7 |
17.32 |
17.6 |
|
MCHC |
g/dl |
32.3-36.7 |
32.5 |
33.2 |
32.9 |
31.3 |
33.098 |
33.1 |
|
RDWc |
% |
- |
18.6 |
18.6 |
18.6 |
19.1 |
18.6 |
20.4 |
|
RDWs |
fl |
- |
39.1 |
39.1 |
38.3 |
39.8 |
37.5 |
42.2 |
|
Platelets |
109/L |
249-611 |
331 |
472 |
292 |
207 |
307 |
295 |
|
MPV |
fl |
- |
8.5 |
8.7 |
10.3 |
8.4 |
9 |
8.3 |
|
PPCT |
% |
- |
0.28 |
0.41 |
0.3 |
0.17 |
0.27 |
0.25 |
|
PDW-cv |
% |
- |
39.1 |
36.2 |
42.3 |
39.1 |
41 |
38.7 |
|
PDW-sd |
fl |
- |
15.6 |
12.3 |
21 |
15.6 |
18.1 |
15.1 |
Table 2 Hematology result
|
Date |
17-Dec-23 |
15-Feb-24 |
15-May-24 |
|||||
|
|
Unit |
Reference Interval |
Utopia |
Ukunda |
Ushabti |
Uele |
Uele |
Uele |
|
ALB |
g/L |
25-44 |
39 |
34 |
37 |
36 |
32 |
38 |
|
ALP |
u/L |
20-150 |
13 |
10 |
10 |
11 |
7 |
7 |
|
ALT |
u/L |
10-180 |
37 |
34 |
39 |
40 |
42 |
41 |
|
AMY |
u/L |
74.96 - 175.24 |
268 |
244 |
258 |
265 |
252 |
222 |
|
TBIL |
μmol/L |
2-10 |
5 |
4 |
5 |
5 |
6 |
7 |
|
BUN |
mmol/L |
2.5-8.9 |
6.2 |
9.4 |
7.4 |
8.1 |
10.5 |
7.9 |
|
CA |
mmol/L |
2.15-2.95 |
2.47 |
2.38 |
2.44 |
2.43 |
2.46 |
2.72 |
|
PHOS |
mmol/L |
1.03-1.61 |
1.54 |
1.68 |
1.63 |
1.37 |
1.46 |
1.29 |
|
CRE |
μmol/L |
27-124 |
142 |
91 |
109 |
9.3 |
93 |
86 |
|
GLU |
mmol/L |
3.3 -6.1 |
7.2 |
8.1 |
9.1 |
7 |
6.5 |
7.7 |
|
NA+ |
mmol/L |
138 -160 |
139 |
133 |
135 |
136 |
140 |
1.43 |
|
K+ |
mmol/L |
3.7 - 5.8 |
5.1 |
5.3 |
5 |
4.4 |
4.7 |
4.2 |
|
TP |
g/L |
54-82 |
62 |
59 |
57 |
57 |
57 |
64 |
|
GLOB |
g/L |
23-52 |
23 |
26 |
20 |
21 |
25 |
26 |
Table 3 Biochemistry result
All newly arrived animals at Dubai Safari Park undergo a mandatory quarantine period during which samples are collected for routine disease screening. This process establishes a baseline health profile, essential for comparison with future tests and for detecting any deviations from normal health. A physical examination, including assessments of body weight, coat condition, and hydration levels, is also conducted to provide a comprehensive evaluation of each animal's general health. Notably, one of the four dogs showed a significant deviation in body weight compared to the others. The health screening and blood test results indicate that all animals have been impacted by prolonged transportation and the new environment. The elevated hematology results, including increased levels of White Blood Cells (WBC), Red Blood Cells (RBC), Hemoglobin (HBG), and Hematocrit (HCT), as well as fluctuations in biochemistry, are evident in the results for all the dogs (Figure 4).
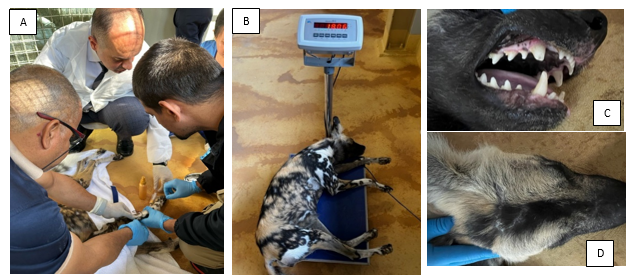
Figure 4 Animal health screening procedure.
(A); Physical examination of the animal including biological sampling, (B); body weight measurement, (C); dental examination, (D); palpation procedure to detect abnormalities such as lumps, swelling or wound post transportation.
Transportation can cause stress in dogs, which might impact their physiological parameters temporarily. Elevated hematocrit values may also signify dehydration, which is linked to a lack of fluids and water loss through breathing while in transportation procedure. Increased rates of respiration and water loss during transportation are caused by heat stress brought on by a high ambient temperature.11 In addition to the transportation procedure, the new environment and social adjustments can also be significant stressors for a newly arrived wild dog in DSP. This stress response can manifest as both behavioral and physiological changes as the dog acclimates to its new surroundings. Such stress is evident in various behaviors, particularly in dogs identified as Uele. Uele exhibits distinct behaviors compared to the other three dogs, demonstrating increased alertness and fearfulness when approaching and accepting food in the dens. To reduce intraspecies aggression during feeding, the keeper places food in multiple locations. As a new addition to the exhibit, Uele is expected to begin marking her territory. Her behavior, however, does not align with Lenkei et al,12 who identified general destructive behaviors such as excessive barking, hyper-salivation, or inappropriate urination and defecation as indicators of stress. Instead, Uele's reactions are more consistent with panic-like responses to separation and extreme noise phobias.
The health screening results for Uele, particularly the rabies antibody titer test, revealed lower levels of antibodies compared to those observed in other dogs. Consequently, Uele is required to undergo an extended quarantine period relative to the other dogs. All dogs arrived with valid vaccination records and subsequently received booster doses during the quarantine period. The immune response to the rabies vaccination was evaluated by measuring serum antibody titer levels. All four dogs demonstrated antibody titers against rabies that exceeded the desired threshold. However, Uele displayed a noticeable decline in body condition due to stress. Her rabies antibody titer was re-evaluated 15 days after the initial measurement and showed a marginal decrease, falling slightly below the acceptable range. Furthermore, her body condition continued to deteriorate. To ensure she received adequate nutrition, she was fed separately from the other dogs.
The decrease in leukocytes, particularly lymphocytes, eosinophils, and neutrophils, in dogs post-transportation likely reflects stress-induced changes in the immune system. Lymphocytes, key players in the immune response, can decrease (lymphopenia) due to stress or immune suppression. Eosinophils, involved in allergic reactions and parasitic infections, may also decrease (eosinopenia) during acute stress or inflammation.13 Barkley MS,14 noted that a decline in neutrophil count (neutropenia) in dogs following transportation can reflect various physiological or pathological responses. Factors such as stress, inflammation, and changes in blood distribution can cause fluctuations in neutrophil levels. Transportation stress may trigger a response leading to neutrophil redistribution to peripheral tissues or the bone marrow, temporarily reducing their presence in the bloodstream and causing a lower observed neutrophil count immediately after transportation. Elevated red blood cell (RBC) count after transportation, reflecting a compensatory immune response to the stress or excitement of transportation.15 The hematology results for Uele, as well as for all the dogs, show elevated levels of White Blood Cells (WBC), Red Blood Cells (RBC), Hemoglobin (HBG), and Hematocrit (HCT), consistent with dehydration. Dehydration reduces plasma volume, concentrating blood components and increasing these parameters without significantly affecting the total red cell mass. Despite these elevated values, the dogs show no behavioral signs of stress and maintain good body condition and appetite. All required quarantine tests, including those for endo- and ecto-parasites, were negative. Consequently, the dogs were stabilized in the animal exhibit.
For dogs traveling internationally, the minimum acceptable rabies antibody titer level is typically defined by the regulations of the destination country or region. The World Organization for Animal Health (OIE) sets the minimum threshold at 0.5 IU/ml.16 Among the four dogs, one (Uele) exhibited a rabies antibody titer below this accepted level. Since all other dogs received the same vaccinations and demonstrated higher immune responses, this suggests that Uele's lower titer may be indicative of a reduced immune response to the vaccine. Individuals with titers ≥ 0.5 IU/mL generally do not need further action, while those with titers < 0.5 IU/mL should receive a booster vaccine. A booster given on days 21 or 28 to ensures long-term immunity.17 On 15 February 2024, during the collection of blood samples, Uele was administered a dose of the monovalent rabies vaccine, “Biocan R.” despite this intervention, subsequent test results indicated an even lower rabies antibody titer than previously observed. Uele was separated from the other three dogs, who were deemed fit for release from quarantine to facilitate their transfer to the main collection facility due to their good health condition and lack of stress behaviors. Although separating wild dogs from their group is typically stressful, Uele showed no significant behavioral abnormalities. In fact, she appeared more relaxed and displayed improved feeding behavior, masticating her food rather than swallowing it whole.
After one month, a follow-up blood sample was collected to reassess Uele’s rabies antibody titer. The results showed a notable improvement in immune response and enhanced rabies titer levels following the separation, improved feeding, and vaccination. Additionally, Uele's overall body condition improved, and her blood tests no longer indicated signs of stress or dehydration. Consequently, Uele was released from quarantine and began a gradual reintegration with the pack, while remaining separated during feeding. Providing her with a larger holding space and exhibit area is expected to further reduce stress and aggression, facilitating a smoother adjustment to her new environment.
The stress response negatively impacts the immune system, reducing resistance to infections. Consequently, immune responses to vaccination are often used as indicators of reactions to infectious challenges. Live attenuated vaccines can effectively stimulate both innate and adaptive immune responses, offering strong antiviral protection.18 Therefore, in this research, a combination of vaccine products showing a low titer of antibodies in wild dogs following vaccination indicates a weaker immune response to the vaccine. This could mean that the vaccine did not elicit a strong immune response or that the animals' immune systems are not responding adequately. However, the monovalent vaccine shows a significant improvement in the antibody titer of wild dogs. Factors affecting antibody titer levels may include the animal's health status, stress levels, and adequacy of the vaccination.
We extended our gratitude to Dubai Safari Park Management, Dubai Municipality for granting approval for conduct of this study and for providing guidance to improve the intellectual content of the subject. We also thank all those who supported the training procedure with their enthusiasm and awesome skill, to lead this training program to have a valuable and great training construction and development for this study.
The authors declared that there are no conflicts of interest.

©2024 Mustafa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.