International Journal of
eISSN: 2574-9862


Research Article Volume 3 Issue 1
Cadi Ayyad University, Morocco
Correspondence: Mohammed Znari, Cadi Ayyad University, Faculty of Science Semlalia, Boulevard Prince Moulay Abdellah, PO Box: 2390, Marrakech, Morocco
Received: October 28, 2017 | Published: January 17, 2018
Citation: Namous S, Znari M. Home range and habitat use of crop-raiding barbary macaques in the upper ourika valley, western high atlas mountains, morocco. Int J Avian & Wildlife Biol. 2018;3(1):36-39. DOI: 10.15406/ijawb.2018.03.00049
Ranging behavior and habitat use reflect the interaction between ecological factors and patterns of the individual behaviors. We investigated these aspects in a crop-raiding group of Barbary macaques (Macaca sylvanus) between July and August 2012 in a degraded and poor-fruit forest environment in the Upper Ourika valley, western High Atlas mountains, Morocco. We evaluated the influences of the spatial distribution of food resources on ranging behavior and habitat use. Total home range size for the studied group was 24 ha and the mean daily path length was 1420 m, much lower than those reported for most of the other macaque species. Barbary macaques did not use their home ranges uniformly; 50% of location records occurred within 25% of their home range. They use differently the various zones of their home range and more intense occupation of around (cliffs and lower small valley) and within field crops. This can be related to the concentration of food resources in these latters.
Keywords: barbary macaque (macaca sylvanus), habitat use, ranging behavior, ourika valley, morocco
Studies of animal ranging behavior and habitat use are of a great importance in investigating the interaction between the ecological factors and the patterns of individual behaviors. Home range size and habitat utilization are assumed to be primarily dependent on the availability, distribution, and quality of food resources.1–6 With generally smaller home ranges, and compared to frugivorous species, folivorous species tend to move over shorter distances each day. In addition, primates can adjust their ranging behavior according to seasonal variations in food availability.7 For instance, the length of daily travel is shortened in some species when high-quality food is rare (e.g., Trachypithecus pileatus,8 Hylobates lar),9 conversely, other species move further to look for high-quality food resources (e.g., Colobus angolensis,10 Trachypithecus leucocephalus).11 Ranging behavior and habitat use may also be influenced by other factors such as rainfall,12 group size,13 forest structure,4 water availability,14 location of dormitory,5 intergroup relationships,15 and parasite avoidance.16 Nevertheless, crop-raiding primates having access to locally concentrate and high-quality food are thought to have also small home ranges that can be affected by the intensity of the human-primate conflict. This could be the case of the Barbary macaque (Macaca sylvanus) in degraded Holm-oak forest patches in its southernmost distribution range in Western High Atlas, Morocco, and where the groups rely on fruit-growing crops for their subsistence during the fruiting season (late spring to mid-autumn).
The Barbary macaque is classified as Endangered on the IUCN Red List of Threatened Species.17 Its distribution range encompasses northwest Africa north of the Sahara desert, from Northern Algeria to Morocco, and Gibraltar.18 They occupy temperate forests including deciduous broadleaf forests, mixed broadleaf and conifer forests, and conifer forests.19 Apart from the numerous behavioral studies, previous investigations of the Barbary macaque have focused on demography19–21 and trophic ecology19–24 and found that the macaques are highly folivorous with young leaves accounting for the bulk of monthly diet almost year-round. However, and apart from a recent work, little quantitative information is available about the ranging behavior and habitat use of this species. Such data are needed to improve our understanding of the biology of M. sylvanus and to provide a comparative perspective on macaque behavioral adaptation. We hypothesize that crop-raiding macaques in degraded forest habitats with fruit-poor environments would exhibit a small home range size due to their dependence on cultivated areas for high-quality food. The purpose of the present work is then to assess the home range size, daily path length, and habitat use by crop-raiding Barbary macaques in the Upper Ourika valley, High Atlas mountains, Morocco, during the fruiting season.
Study area and animals
The present study was conducted from March 2012 to September 2012 at Setti Fatma (7°52’80,9”W, 31°13’33,8”N), Upper Ourika valley, western High Atlas, Morocco (Figure 1). The study area consists of limestone cliffs with elevations ranging from 900 to 2500 m above sea level.25 The vegetation profile is forest (mostly Holm-oak, Quercus ilex) with significant changes at different levels due to differences in temperature, humidity, and soil quality26 (Figure 1).
Figure 1 Map of Morocco showing the study area in Upper Ourika valley, Western High Atlas range, Morocco.
Data collection and analysis
Because of rugged terrain, which was frequently difficult, it was not possible to conduct full-day consecutive behavioral sampling most of the time. In this study, we made behavioral observations on Setti Fatma group in summer 2012 for 13 full days (7 in July and 6 in August) and 24 partial days (11 in July and 13 in August). For full-day follows, we started to observe the macaques once they left their sleeping sites and finished when they returned to them. For partial-day follows, observation began when the animals were first seen and ended when they were out of sight or went to their sleeping sites. These partial-day follows were variable and lasted in average 3.57±1.49h (range = 2-6.5). We registered the location of the focal group every 30 min using a GPS; and the geo-referenced data-points were plotted later on a topographic map (1:50,000). During the study period, we collected 173 location records. The distance between the observer and the detected group varied between 25 to 70m. Among the detected groups, the locations of feeding groups were considered to assess their spatial distribution within the study area. We estimated the home range size and pattern of range use by superimposing a grid of 100×100 m quadrats (1 ha) on the map of the study area. The home range size was considered as the total area of all unique quadrats penetrated during the study period. We expressed the pattern of range use as the percentage of the total location records that fell into each 1-ha quadrat of the grid. We defined a core area as the cumulative area of all quadrats within the home range within which macaques resided more than the expected uniform percentile value [25]. We ranked the overall intensity of quadrat use across the home range among five use-intensity categories: ≥ 6.0% total use, >3.0% to < 5.9% use, > 0.1% to < 2.9% use, and no use.
Analyses of daily travel patterns were based only on full-day follows. The cumulative straight-line distances between successive chronological locations for each day, allowed assessing daily path length. We expressed the habitat use as the percentage of locations recorded in the study site during summer period. We used Chi-square tests to compare the observed frequencies of quadrat use to the frequencies expected randomly, using the total number of quadrats entered, the total number of location records and subsequent analysis because too few records were collected during these months. All tests are two-tailed, with a significance level of p<0.05.
Home range size and daily travelling length
During the study period, Barbary macaques entered 26 different 1-ha quadrats corresponding to the total home range size (26 ha). In this study, we recorded 13 full-day follows for the studied group. On average, the group traveled 1420±306.2 m per day (range: 1050-1940m) (Figure 2).
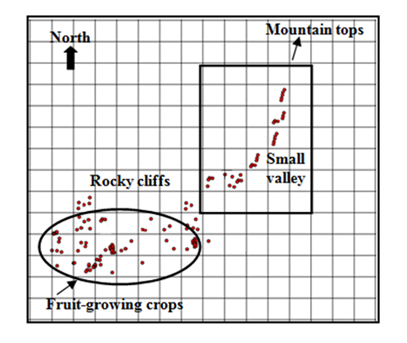
Figure 2 Home range of the Barbary macaque study group, and overall the intensity of one-ha quadrats use and distribution of group feeding sites (fruit-growing crops and small valley) in the home range, in Upper Ourika valley, Western high Atlas, Morocco.
Home range and habitat use
The intensity of site use varied considerably. Macaques penetrated several quadrats only once or twice, whereas the mostly used quadrats accounted for 25% of all recorded locations. There was a significant difference between observed and expected frequencies of quadrat use (χ2=38.64, df=25, p<0.05), suggesting that the macaques select some areas of their habitat. Fifty percent of location records occurred in only seven quadrats. Ten core quadrats together accounted for 70% of all location records (Figure 2). The distribution of group feeding in the home range also showed a similar pattern (Figure 2). During the study period, we recorded group feeding, which occurred within 17 quadrats of home range. 75% of group feeding occurred in only 8 quadrats. There was a significant and positive correlation between the intensity of quadrat use and the group distribution (rs =0.897, n=17, p<0.001). There was a highly significant difference in the use of different zones of the study area (χ2=23.90, df= 3, p<0.001). Field-crops (including Walnut trees) accounted, on average, for 43.7% of the total of the location records, whereas small valleys, cliffs-mount sides and higher valleys averaged, 19.8%, 10.2% and 26.3%%, respectively. Barbary macaques were never seen to use the valley basin during the study period.
Regardless of the season and the duration of the study period, the home range figures of 24ha and the mean daily path lengths of 1420m we found for Barbary macaques in Upper Ourika valley, are much smaller than those observed in other macaques: M fascicularis: 93ha and 3346m,26 M fuscata: 370ha and 1200-2000m,27,39 M mulatta: 1600ha and 1050-3500m,29,30 M nemestrina: 60-828ha and 825-2964m,31,32 M nigra: 156-406ha and 2388m.33 In M assamensis, home range was also larger (53-65ha) but with a shorter path length of only 590-782m.6 In the Middle Atlas mountains, the mean day range length in Barbary macaque were found to increase with the intensity of pastoralism, from 1604m to 2731m, respectively in undisturbed or moderately impacted forest and highly disturbed forests.7 The lower path length in the studied macaque’s group may be related to our study groups’ greater reliance on crops. In upper Ourika valley, fruits and seeds accounted for nearly 70% of the Barbary macaque’s diet whereas core plants only constituted 30% of the diet (in preparation). Barbary macaques in upper Ourika valley consume less fruits (50%) than M fascicularis: 66.7%,34 M nemestrina: 74%,33 or M nigra: 66%.33 Unlike fruits, leaves are an abundant and uniformly distributed food resources but with much less nutritional and energetic values.35 Consequently, these macaques travel much less widely in search of food. Concurrently, group size would be a main factor affecting ranging behavior in primates, with large-sized groups typically with larger home ranges and longer daily path lengths.36–38 At only 32-46 individuals,39 the studied group is smaller than those observed in other Macaca species: M fascicularis (66 individuals, Jamieson, 1998), M fuscata (41 individuals),28 M nemestrina (49-81 individuals),31 or M nigra (42-97 individuals).33
Barbary macaques in the upper Ourika valley used large parts of their home range very infrequently, concentrating their activities around and within crops. This indicated that the differential utilization of the group’s home range may be related to the distribution of key foods. Predation risk and food availability are expected to be the major factors influencing habitat use in primates.40,41 We found that Barbary macaques spent the major part of daytime around and within cultures, but still relatively fearful towards the local farmers. In fact, the occurrence of Barbary macaques in the cultivated areas is most of the time tolerated in the upper Ourika valley, but when crop-raiding becomes more intense, especially in late spring-early autumn, this usually results in chasing macaques or, in the very rare and worse cases, are even injured or killed. So, out of the feeding periods, the Barbary macaques would prefer the higher zones for greater security (i.e., rocky cliffs, hilltops and higher valleys). A similar behavior was also noted in Nonggang, China, in the Assamese macaques, but different of their sympatric Rhesus macaques (M mulatta) that are less fearful of the presence of humans.6 Given the highly degraded status of forest patches (especially Holm oak), and as the herbaceous stratum progressively disappeared during summer, Barbary macaques show a more and more high dependence on cultivated plants. Predation risk and food availability are thought to be the major factors influencing habitat use in primates.40,41 We found that Barbary macaques spent 46% of daytime and field-crops. While showing a certain tolerance to the presence of macaques in their field crops, local farmers avoid sharing their crops at an unaccepted level. When crop-raiding becomes more intense, particularly during summer-autumn, they often chase them away from their field crops, (usually by throwing stones at them or by using guard dogs), but cannot shoot them as the macaque population is managed by the Toubkal National Park administration. In addition, Barbary macaques constitute one of the main tourist attractions in this area.
Food resources are also likely to be important determinants of habitat use in Barbary macaques in Setti Fatma. One of the most important food species, the cultivated walnut, Juglans regia, is abundant on lower hillsides and in the high small valley over waterfalls (Namous et al., in prep.). Thus, the higher small valley is both a low-risk and a quite food-rich habitat during the summer period. On the other hand, the lower field-crops are more food-rich but relatively risky habitats. Although there are marked seasonal changes in macaques’ foods (Namous et al., in prep.), the Barbary macaques are highly dependent on field crops most of the year, from late spring to mid-autumn. This period coincides with birth and lactation season during which nutritional and energy requirements are particularly high. More data are required for evaluating the influence of temporal variability in food resources on habitat use of Barbary macaques in the Upper Ourika habitats.
The home range of the Setti Fatma group, Upper Ourika valley, western High Atlas mountains, Morocco, stretched over an estimated area of only 24ha used non-uniformly and occupying mainly cliffs and valleys in the vicinity of crop fields. As expected the Barbary macaques had a daily path length of less than 1500m, which is much shorter than those of other macaque species in relation to the high dependence on the crops.
We are indebted to Pr Jan Siess and his students Manon and Armand for their excellent assistance during the field work. This work was partly funded by the People’s Trust for Endangered Species (PTES, London), from 2012 to 2014.
There is no conflict of interest among the authors.

©2018 Namous, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.