International Journal of
eISSN: 2574-9862


Research Article Volume 5 Issue 2
1Bahir Dar University College of Agriculture and Environmental Sciences, School of Fisheries and Wildlife Management, Ethiopia
2Dean of Bahir Dar University College of Agriculture and Environmental Sciences, Ethiopia
3Dean of Biology Department at Bahir Dar University, Ethiopia
Correspondence: Shimelis Aynalem, Dean of Bahir Dar University College of Agriculture and Environmental Sciences, Ethiopia, Tel 0918008194
Received: November 02, 2020 | Published: December 31, 2020
Citation: Desalegn T, Aynalem S, Tassie N. Diversity, abundance and habitat association of avifauna in Menagesha Amba Mariam and Gara Medhanialem forest, in Oromia Region, Ethiopia. Int J Avian & Wildlife Biol. 2021;6(1):1-10. DOI: 10.15406/ijawb.2020.05.00175
The study was conducted from August 2018 to March 2019 by considering the wet and dry seasons. The aim of this study is to investigate diversity, abundance and habitat association of bird species in Menagesha Amba Mariam and Gara Medhanialem forest. Stratified random sampling technique was employed. Point transect techniques was applied in forest and woodland habitats and line transects technique was used in the farmland habitat. Shannon diversity index and chi-square test were employed for data analysis. A total of 112 bird species that belong to 16 orders and 45 families were recorded. Three are endemic to Ethiopia and Twelve species endemic to both Ethiopia and Eritrea. The highest species diversity (H'=3.60) was recorded from the forest habitat and the lowest (H'=2.95) in the farm land. The association of bird species with habitat was statistically significantly different in wet season (χ2= 1702.9, df=180, p<0.001) and in dry season (χ2=1497.5, df=172, p<0.001). Availability of food and nesting sites were the major factors determining the diversity of birds. Government officials and local community participation is needed for conservation of Menagesha Forest resources.
Keywords: bird species, habitat type, Menagesh forest and species similarity
Ethiopia is one of the top 25 biodiversity rich countries in the world and hosts two of the African biodiversity hotspots, namely the eastern Afromontane and the horn of Africa hotspots.1 The two hotspots comprise highland massive surrounded by arid lowland and contains various wildlife and their habitats ranging from 116 meters (b.s.l) in the Afar Depression to 4620 meters (a.s.l) at Ras Dajen2 It is a significant regional center for biological diversity due to its wide ranges of elevation and wider variations of topography.3 The major geographic features are massive highland mountains and plateau divided by the Great Rift Valley and surrounded by lowlands along the periphery. The Great Rift Valley runs from Ethiopia extending in to Tanzania and Great Lakes region of Africa, in the south creating a vast depression.2 Those natural factors described above powerfully influenced Ethiopia’s extraordinary range of terrestrial and aquatic ecosystems, and contributed to be endowed with a high diversity and rate of endemism.4 Consequently, the subsistence of such diverse ecosystems has gifted Ethiopia to be known for its avifauna diversity.
Worldwide, there are over 10, 000 species of birds5 that belonging to 204 families.6 More than 50 percent of the extant avian species belong to the order Passeriformes. There are over 2,341 bird species in Africa.7 Ethiopia has 867 species of birds, where 19 are endemic, 38 are globally threatened, 1 is introduced and 13 species restricted to the geographical region of Ethiopian and Eritrean highlands and thus, shared only by Ethiopia and Eritrea.8
Birds perform several ecological roles for ecosystem functioning and produce great benefits for human population in some circumstances.9 In the last decades, many studies have assessed an array of ecosystem functions mediated by birds, such as pollination,10 control of insect populations11 in different ecosystems. About half of the plants are clearly adapted to bird pollination as well as indicates environmental health and they have been considered as environmental indicator species.12 They are also called bio indicators of climate change and water quality and also potential tourist attractions.13 The abundance of birds as bio indicator species showed strong and significant positive correlations with both tree abundance and cover in each vegetation type.14
In Ethiopia, there are many designated protected areas (PAs) including National Parks, Wildlife Reserves, Controlled Hunting areas, Priority Forest area, Biosphere Reserves and Community Conservation Areas.15 Protected areas have a vital role for conservation and protection of biodiversity. Before 1970 Ethiopia has two protected areas but know it has 67 protected areas to conserve natural ecosystems and wildlife heritage.16 There are 24 National Parks, 3 Biosphere Reserve, 2 Wildlife Sanctuaries, 6 Wildlife Reserve, 5 Community Conservation Areas, 21 Controlled Hunting Areas and 6 Open Controlled Hunting areas.17
Menagesha Amba Mariam and Gara Medhanialem forest is one of the conservations protected area in Oromia Region Ethiopia is rich in flora and fauna.18 This forest also one of the important bird area in Ethiopia. However, there is little scientific investigation on the avifauna of state forest. Therefore, this study helps to fill the gap on the diversity, abundance and habitat association of avifauna at Menagesha Amba Mariam and Gara Medhanialem forest.
Description of the study area
This study was carried out at Menagesha Amba Mariam and Gara Medhanialem Forest. The forest is found in the central part of Ethiopia 30km far to the west of Addis Ababa, the capital city of Ethiopia neighbored by Sebeta and Holeta towns. It is located between 9°01'-9o02'N latitude and 38o34'-38o35'E longitude in the Oromia regional state, Ethiopia, between an altitudinal range of 2200- 2910m. The total area of the State Forest is 207 ha (Figure 1) of which 31% is under cultivation for growing both annual and perennial crops, 28% woodland while 41% is forest. The study area flanked by Mt. Wochacha to the south, Kolbo Kebele to the north and Sademo town to the west and Gefersa town to the east.
Flora and fauna
There are 32 plant species, of which African olive (Olea europaea) cover (17.7%) followed by African juniper (Juniperus procera) (15.8%) Blue bitter-berry (Olinia rochetiana) (11.3%) and Tree heath (Erica arborea) (9.73%).18
The study area is the habitat of numerous wild animals including, Olive baboon (Papio Anubis), Black and White Colobuses (Colobus guereza), Spotted hyena (Crocuta crocuta) and Grivet monkey (Chlorocebus aethiops).
Climate
There is no available metrological data in Menagesh Amba Mariam and Gara Medhanialem Forest. But there are two stations near the forest, Sebeta and Addis Ababa (Ayertena) where weather data was obtained. Approximately the climate of the area was the mean of the two stations. The rainfall and temperature condition of the area was defined based on the data collected from 2007-2017 by the National Metrological Agency (NMA). According to the data from NMA, the result of the analysis showed that the mean monthly maximum and minimum temperature of the study area is 23.5ºC and 8.6ºC respectively. The hottest month is March with maximum temperature of 26.13ºC, followed by May 25.19ºC and the coldest month is December with a temperature record of 8.5ºC (Figure 2).
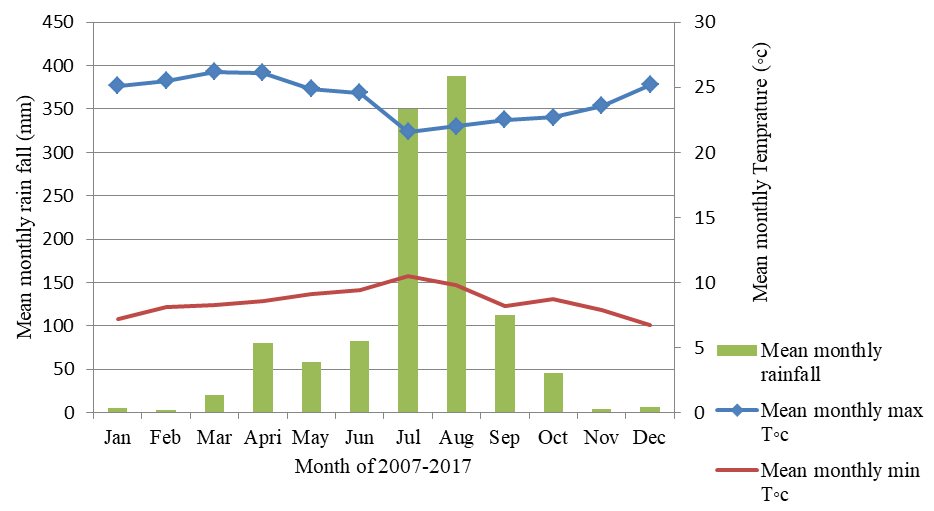
Figure 2 Mean monthly maximum and minimum temperature and rain fall of Menagesha Amba-Mariam and Gara-Medhanialem Forest (2007-2017).
The mean annual rainfall is 1050mm and the mean annual temperature is 23.8ºC. The rain mainly falls from June to September, with the highest concentration in July and August. June to December is comprises the wet season and December to March the dry season.
Materials used
Materials used during the field survey include10×50 field binoculars; Geospatial Positioning System (GPS), sound recorder, digital camera and bird guide books.19, 20
Sampling design and data collection
A preliminary survey was carried out in July, 2018 to gather basic information about the study site. The physical features of the study area were assessed using ground survey. The actual study was carried out from August, 2018 to March, 2019 by considering both the wet and the dry seasons. The wet season data covers from August to November and the dry season was from December to March. In this study Stratified random sampling design was employed, since the study area was not uniform in terms of habitat types. The area was stratified into three habitats types (Forest, Farmland and Woodland).
A total of 25 sampling plots were purposively placed covering the whole study area 207 hectare. Different types of sampling plots were assigned for each habitat. The sampling plots were selected based on the area proportion of each representative habitat. Each study plot had consisted of 9-point counts. Each plot measures 3.24 hectare to cover 39% of the study area. The radius of each point was 30m and there was 200m distance between plots to avoid double counting of individual birds.21
In forest area and wood land point transect technique was used while in the farmland the researcher used line transect count techniques. There were ten plots in the forest, seven plots in woodland and eight plots in the farm land (Table 1). There were 16 lines transect to record the abundance of birds in the farmland.
Habitat type |
Forest |
Woodland |
Farmland |
Area coverage in hectare |
84.5ha |
58.5ha |
64ha |
Proportion of sample |
41% |
28% |
31% |
Number of plots |
10 |
7 |
8 |
Number of point counts for each plot |
9 |
9 |
- |
Total number of point count |
9×10=90 |
7×9=63 |
- |
Total number of lines transect |
- |
- |
8×2= 16 |
Table 1 Habitat classification and allocation of plots based on area coverage
In each site, bird observation was carried out twice daily 6:30 AM to 10:00 AM in the morning and 4:00 PM and 6:00 PM in the afternoon. Birds were counted as seen, heard and when flight on the sky. Field data collection was carried out for three days per week depending on the weather conditions and time of the day when bird species were active. Bird identifications and counting of individuals was conducted by direct observations. Observations were made by standing in the middle of the sampling point and observing 360° round quietly and gently. Upon reaching a point, 2-5 minutes was stayed for the birds to settle in case of any disturbances.
The data was analyzed by using simple descriptive statistics; bird abundance and diversity was calculated using Shannon-Wiener Diversity Index which provides an account for both abundance and evenness22 and the data was analyzed using SPSS version 20.0 software.
Shannon-Wiener Diversity Index (H') was calculated as:
Diversity index ………. (1)
Where,
H' = the Shannon-Wiener Index of Diversity
Pi = the proportion of the species relative to the total number of species
ln= Indicates natural logarithm
Sorensen similarity index (S) used to measure species similarity of different habitat types. It is equal to 1 in case of complete species similarity between three habitats and 0 if species of two habitat types are dissimilar.23
Sorensen similarity index (S) is computed as:
……………. (2)
Where:
A = Number of species that occur in a site A
B = Number of species that occur in a site B
C = Number of common species that occur in a site A, B and D
D=Number of species that occur in a site D
Species evenness is often assessed by Shannon equitability index (E) which is calculated by:
……. (3)
Where; H'max = ln(S) = natural logarithm of the total number of species (S) in each habitat.24 E values ranges from 0 to 1, in which 1 indicates complete evenness.
Richness index (RI) of each species was determined using the formula:
…………………… (4)
Where, S = Number of species in each habitat, ln = Natural logarithm, pi= Number of individual species in each habitat
Relative abundance of avian species was determined by using encounter rates that give crude ordinal scales of abundance (abundant, common, frequent, uncommon and rare).25
Encounter rate was calculated for each species by dividing the number of birds recorded by the number of hours spent searching, in order to get a figure of birds per hour for each species (Table 2).
Abundance category |
Abundance score |
Ordinal scale |
<0.1 |
1 |
Rare |
0.1-2.0 |
2 |
Uncommon |
2.1-10.0 |
3 |
Frequent |
10.1-40.0 |
4 |
Common |
>40.0 |
5 |
Abundant |
Table 2 Encounter rates used to give a crude ordinal scale of abundance
Relative abundance= Total number of individuals of one species (n)/ Total no. of individuals of all species (N) ×100 …… (5)
Chi-square test (χ2) was employed to see the association of birds among the three habitat types (Forest, Woodland and Farmland) and Chi-square (χ2) goodness of fit was used for test abundance of bird species in the study area.
Abundance and composition
A total of 112 avian species that belongs to 16 and 45 family’s orders were recorded during the present study period (Table 2). Majority of 67% them are under order Passeriformes. The highest number of species was recorded in families: Accipitridae, Columbidae, Ploceidae and Fringillidae. The lowest number of species was recorded from seventeen Families, 1 species each. Yellow-fronted Parrot (Poicephalus flavifrons), Abyssinian Catbird (Parophasma galinieri) and Abyssinian Woodpecker (Dendropicus abyssinicus) are endemic to Ethiopia. Twelve species are endemic to both Ethiopia and Eritrea. Five species near endemic to Ethiopia and one species is Intra-Africa migrant were recorded, but the remaining 91 species were residents (Table 3).
|
Order |
Family |
Common name |
Scientific name |
|
Accipitriformes |
Accipitridae |
African Fish-Eagle |
Haliaeetus vocifer** |
|
African Hawk-Eagle |
Haliaeetus spilogaster+ |
||
|
Augur Buzzard |
Buteo augur** |
||
|
Bateleur |
Terathopius ecaudatus** |
||
|
Black-chested Snake-Eagle |
Circaetus pectoralis** |
||
|
Hooded Vulture |
Necrosyrtes monachus* |
||
|
Tawny Eagle |
Aquila rapax* |
||
|
Verreaux's Eagle |
Aquila Verreauxii* |
||
|
White-headed Vulture |
Trigonoceps occipitalis* |
||
|
Yellow-billed Kite |
Milvus aegyptius+ |
||
|
Anseriformes |
Anatidae |
Egyptian Goose |
Alopochen aegyptiaca** |
|
Apodiformes |
Apodidae |
Nyanz Swift |
Apus niansae+ |
|
Bucerotiformes |
Bucerotidae |
African Grey Hornbill |
Tockus nasutus+ |
|
Red-billed Hornbill |
Tockus erythrorhynchus** |
||
|
Coliiformes |
Collidae |
Speckled Mousebird |
Colius striatus** |
|
Coraciiformes |
Meropidae |
Blue-breasted Bee-eater♦ |
Meros lafresnayii+ |
|
Little Bee-eater |
Merops pusillus* |
||
|
Columbiformes |
Columbidae |
African Olive Pigeon |
Columba arquatrix* |
|
Blue-spotted Wood Dove |
Turtur afer** |
||
|
Dusky Turtle Dove |
Streptopelia lugens** |
||
|
Laughing Dove |
streptopelia senegalensis** |
||
|
Red-eyed Dove |
Streptopelia semitorquata+ |
||
|
|
Lemon Dove |
Aplopelia larvata* |
|
|
Speckled Pigeon |
Columba guinea** |
||
|
Tambourine Dove |
Turtur tympanistria* |
||
|
White-collared Pigeonϫ |
Columba albitorques** |
||
|
Falconiformes |
Falconidae |
Lanner Falcon |
Falco biarmicus** |
|
Galliformes |
Phasianidae |
Erckel's Francolin♦ |
Pternistis erckelii** |
|
Gruiformes |
Rallidae |
Rouget's Rail ϫ |
Rougetius rougetii** |
|
Musophagiformes |
Musophagidae |
White-cheeked Turaco♦ |
Tauraco leucotis** |
|
Passeriformes |
Alaudidae |
Erlanger's Lark ϫ |
Calandrella erlangeri** |
|
Buphagidae |
Red-billed Oxpecker |
Buphagus erythrorhynchus** |
|
|
Cisticolidae |
Buff-bellied Warbler |
Phyllolais pulchella* |
|
|
Grey-backed Camaroptera |
Camaroptera brachyura** |
||
|
Red-faced Cisticola |
Cisticola erythrops* |
||
|
Tawny-flanked Prinia |
Prinia subflava+ |
||
|
Corvidae |
Cape Rook |
Corvus capensis+ |
|
|
Fan-tailed Raven |
Corvus rhipidurus** |
||
|
Pied Crow |
Corvus albus+ |
||
|
Thick-billed Ravenϫ |
Corvus crassirostris+ |
||
|
Estrildidae |
African Firefinch |
Lagonosticta rubricata** |
|
|
Bronze Mannikin |
Spermestes cucullata* |
||
|
Red-cheeked Cordon-bleu |
Uraeginthus bengalus** |
||
|
Red-billed Firefinch |
Lagonosticta senegala** |
||
|
Yellow-billed Waxbill |
Coccopygia quartinia** |
||
|
Fringillidae |
African Citril |
Serinus citrinelloides** |
|
|
Brown-rumped Seadeater |
Serinus tristriatus** |
||
|
Streaky Seedeater |
Serinus striolatus** |
||
|
Stripe-breasted Seedeater |
Serinus reichardi** |
||
|
Yellow-crowned Canary |
Serinus Serinus flavivertex+ |
||
|
Yellow-fronted Canary |
Serinus mozambicus+ |
||
|
Hirundinidae |
Rock Martin |
Ptyonoprogne fuligula* |
|
|
Lesser Striped Swallow |
Cecropsis abyssinica** |
||
|
Laniidae |
Common Fiscal |
Lanius collaris** |
|
|
Locustellidae |
Cinnamon Bracken Warbler |
cinnamomeus+ |
|
|
Macrosphenidae |
Northern Crombec |
Sylvietta brachyura+ |
|
|
Malaconotidae |
Ethiopian Boubou |
Laniarius aethiopicus** |
|
|
Back-crowned Tchagra |
Tchagra senegalus* |
||
|
Monarchidae |
Abyssinian Slaty Flycatcherϫ |
Melaenornis chocolatinus+ |
|
|
African Dusky Flycatcher |
Muscicapa adusta+ |
||
|
African Paradise Flycatcher |
Trochocercus cyanomelas** |
||
|
Motacillidae |
White Wagtail♣ |
Motacilla alba* |
|
|
Mocking Cliff Chat |
Thamnolaea |
||
|
Muscicapidae |
cinnamomeiventris** |
||
|
Moorland Chatϫ |
Cercomela sordida** |
||
|
Ruppell's Black-Chat |
Myrmecocichla melaena+ |
||
|
Ruppell's Robin-Chat ϫ |
Cossypha semirufa** |
||
|
Nectariniidae |
Beautiful Sunbird |
Cinnyris pulchellus* |
|
|
Malachite Sunbird |
Nectarinia famosa** |
||
|
Scarlet-chested Sunbird |
Chalcomitra senegalenis** |
||
|
Shining sunbird |
Cinnyris habessinicus** |
||
|
Tacazze Sunbird |
Nectarinia tacazze** |
||
|
Variable Sunbird |
Cinnyris venustus** |
||
|
Oriolidae |
Abyssinian Oriole |
Oriolus monacha** |
|
|
Black-headed Oriole ϫ |
Oriolus larvatus+ |
||
|
Paridae |
White-backed Black Tit ϫ |
Parus leuconotus** |
|
|
Passeridae |
Swainson's Sparrow♦ |
Passer swainsonii** |
|
|
Phylloscopidae |
Brown Woodland Warbler |
Phylloscopus umbrovirens+ |
|
|
Ploceidae |
Baglafecht Weaver |
Ploceus baglafecht** |
|
|
Fan-tailed Widowbird |
Euplectes axillaris** |
||
|
Little Weaver |
Ploceus luteolus+ |
||
|
Red-collared Widowbird |
Euplectes ardens* |
||
|
Spectacled Weave |
Ploceus ocularis+ |
||
|
Village Weaver |
Ploceus cucullatus+ |
||
|
Vitelliane Masked Weaver |
Ploceus vitellinus+ |
||
|
Yellow Bishop |
Euplectes capensis** |
||
|
Yellow-crowned Bishob |
Euplectes afer* |
||
|
Yellow-mantled Widowbird |
Euplectes macroura* |
||
|
Pycnonotidae |
Common Bulbul |
Pycnonotus barbatus** |
|
|
Sturnidae |
Greater Blue-eared Starling |
Lamprotornis chalybaeus+ |
|
|
Red-winged Starling |
Onychognathus morio** |
||
|
Sylviidae |
Abyssinian Catbird♠ |
Parophasma galineri** |
|
|
Timaliidae |
White-rumped Babbler♦ |
Turdoides leucopygia** |
|
|
Turdidae |
African Thrush |
Turdus pelios+ |
|
|
Mountain Thrush |
Turdus abyssinicus** |
||
|
Groundscraper Thrush |
Psophocichla litsitsirupa** |
||
|
White-winged Cliff Chat ϫ |
Thamnolaea semirufa+ |
||
|
Viduidae |
Pin-tailed Whydah |
Vidua macroura+ |
|
|
Village Indigobird |
Vidua chalybeata+ |
||
|
Zosteropidae |
Abyssinian White-eye |
zosterops abyssinicus** |
|
|
Yellow White-eye |
Zosterops senegalensis** |
||
|
Pelecaniformes |
Threskiornithidae |
Hadada Ibis |
Bostrychia hagedash+ |
|
Wattled Ibisϫ |
Bostrychia carunculata** |
||
|
Scopidae |
Hamerkop |
Scopus umbretta+ |
|
|
Piciformes |
Indicatoridae |
Lesser Honeyguide |
Indicator minor* |
|
Picidae |
Abyssinian Woodpecker♠ |
Dendropicus abyssinicus** |
|
|
Cardinal Woodpecker |
Dendropicos fuscescns* |
||
|
Grey-headed Woodpecker |
Dendropicos spodocephalus+ |
||
|
Psittaculidae |
Nubian Woodpecker |
Campethera nubica* |
|
|
Psittaciformes |
Black-winged Lovebirdϫ |
Agapornis taranta** |
|
|
Yellow-fronted Parrot♠ |
Poicephalus flavifrons** |
||
|
Trogoniformes |
Trogonidae |
NarinaTrogon |
Apaloderma narina* |
Table 3 Bird species recorded during the wet and the dry seasons at Amba-Mariam and Gara-Medhanialem forest
*=Dry; +=wet; **both Wet and Dry; ♠ Endemic; ϫ Endemic to Ethiopia and Eretria; ♦ Near Endemic; ♣ Inter Africa migrant; unmarked species are resident birds)
Forest habitat had high number of individuals in both seasons followed by woodland and least number of individuals recorded at the farmland habitat (Figure 3).
The Chi-square test for abundance of birds during the study period in the two seasons showed that there was statistically significant difference between observed and expected counts of each species. The abundance of bird chi-square test during the wet season (χ2=1504.8, df=90, p<0.001) and the dry season (χ2=1842.7, df=86, p<0.001).
During the wet and dry seasons, 90 and 81 bird species were recorded, respectively. Among them 59 bird species were common for both seasons, while 22 and 31 species were exclusively recorded during the wet and the dry seasons, respectively (Table 2).
The dominant bird species recorded in the study area were Brown-rumped Seadeater (n=198), Swainson's Sparrow (n=127), Ruppell's Robin-Chat (n=122) and Abyssinian Oriole (98).The number of bird species in the forest habitat was higher compared to the Woodland and Farmland (Figure 4).
Species diversity
The highest species diversity (H'=3.60) of birds was recorded from the forest habitat during the wet season, followed by woodland habitat (H'=3.49), whereas the least diversity (H'=3.37) was recorded from the farmland habitat during the wet season. During the dry season, the highest species diversity was recorded in the forest habitat (H'=3.36) and the least was in the farmland (H'=2.95). During the wet season, the highest and the lowest species evenness was recorded in woodland (0.95) and farmland (0.86) habitats, respectively. The woodland habitat had high species evenness but the least evenness was registered from the farmland habitat in both seasons.
The highest richness index was recorded (RI=7.61) from the forest habitat and the least richness (RI=4.79) was recorded from the farmland habitat (Table 4). The highest number of species was recorded in the forest habitat in both seasons. A total of 50 and 44 species were recorded in the forest habitat in wet and dry seasons, respectively. But the smallest number of bird species was recorded from the farmland habitat 29 during dry season.
Habitat |
Season |
No. of species |
Abundance (no. of individuals) |
D |
RI |
H' |
H'/Hmax |
Wet |
50 |
625 |
0.959 |
7.61 |
3.6 |
0.92 |
|
Forest |
Dry |
44 |
387 |
0.943 |
7.22 |
3.36 |
0.89 |
Wet |
40 |
445 |
0.958 |
6.39 |
3.49 |
0.95 |
|
Woodland |
Dry |
35 |
384 |
0.946 |
5.71 |
3.24 |
0.91 |
Wet |
40 |
433 |
0.947 |
6.42 |
3.37 |
0.91 |
|
Farmland |
Dry |
29 |
342 |
0.921 |
4.79 |
2.95 |
0.86 |
Table 4 Bird species abundance, diversity, richness and evenness during the wet and dry season
H',Shannon-weiner Index; D,1-∑pi2; RI=Richness Index; H'/Hmax=Evenness; Hmax=Ln(S); S, The total number of species
Relative abundance of birds in wet and dry seasons
During the study period 34, 18 and 22 species were uncommon; 15, 21 and 17 species were frequent and 1 species was recorded common in each habitat during the wet season. Likewise, during the dry season 26, 12 and 14 species were uncommon; 17, 22 and 14 species were frequent; 1 species was common at each habitat (Table 5).
Habitat |
Season |
Uncommon |
Frequent |
Common |
Wet |
34 |
15 |
2 |
|
Forest |
Dry |
26 |
17 |
1 |
Wet |
18 |
21 |
1 |
|
Woodland |
Dry |
12 |
22 |
3 |
Wet |
22 |
17 |
1 |
|
Farmland |
Dry |
14 |
14 |
2 |
Table 5 Number of bird species between different habitat relative abundance categories
There was 40% avian species similarity between forest and woodland habitats during the wet season. But 8.2% avian species similarity was observed between farmland and forest habitat during the dry season. Similarity decrease as we go from farmland with woodland, farmland with woodland and farmland with forest, respectively in both seasons (Table 6 & 7). The three habitat types had 9.2% and 5.6% avian species similarity during the wet and dry seasons, respectively.
Habitats |
Common species |
Similarity index |
Farmland with Forest |
9 |
0.2 |
Farmland with Woodland |
10 |
0.25 |
Forest with Woodland |
19 |
0.4 |
Forest with woodland with Farmland |
4 |
0.092 |
Table 6 Sorensen similarity index (S) of bird species between different habitats during the wet season
Habitat |
Common species |
Similarity index |
Farmland with Forest |
3 |
0.082 |
Farmland with Woodland |
5 |
0.156 |
Forest with Woodland |
13 |
0.329 |
Forest with woodland with Farmland |
2 |
0.056 |
Table 7 Sorensen similarity index (S) of bird species in different habitats during the dry season
The chi-square test for habitat association of bird species during the study period in the two seasons with the three habitat types (forest, woodland and farmland) showed that there was statistically significantly different, in wet season (χ2= 1702.9, df =180, p<0.001) and in dry season (χ2=1497.5, df=172, p<0.001) across in the studied habitats throughout the study period.
A total of 112 avian species that belong to 45 families were recorded during the present study period with large proportion of species recorded under order Passeriformes. In line with this study, Kalkidan & Afework26 and Girma et al.27 found that Passeriformes was the most dominant order. Likewise, the recent report by Seyoum et al.28 also pointed out that order Passeriformes was numerically the dominant order represented with 22 species which accounts for 44% of the identified species. Ploceidae and Colombidia were the most dominant families in the present study. This might be due to habitat quality, flower composition, high productivity of fruits, availability different nesting vegetation’s.29 Likewise, Gloria30 reported that birds select habitats that fit their requirements for successful reproduction and survival although some generalist species may utilize several habitats.
The diversity of birds varied within the three habitat types, which could be due to vegetation type, foliage height, nesting availability and floristic composition of the area. Similarly, Waterhouse et al.31 showed that the food and cover requirement of bird species is determined mainly by the vegetation structure and composition. Soderstrom & Part32 in Sweden reported that birds often prefer to utilize multiple habitats and depend on the quality and productivity of habitats in terms of food availability, shelter and breeding areas in order to maintain viable population. In addition to these Akobu et al.33 reported that the distribution of nectarivorous birds positively associated with habitat of complex vegetation in the Assop forest that had higher plant diversity.
In this study the diversity, abundance and habitat association of birds are determined in terms of water availability, feeding requirement, floristic composition and nesting habitats. Similarly, Rodriguez-Estrella34 reported that the pattern of bird distribution is strongly related to environmental factors (topography, food and habitats) and human interventions. Meseret & Solomon35 also supported the present study that wild species prefer to habitats where their ecological needs are fulfilled.
The highest numbers of bird species were observed in the forest habitat during both the wet and dry seasons. Kalkidan & Afework26 in Entoto Natural Park and Escarpment also reported that the highest numbers of avian was observed in the forest habitats. This is possibly due to the diversity of vegetation that provided for different bird species. In addition to that Hailemariam et al.36 suggested that species composition and abundance of birds were mainly determined by vegetation structures that used as breeding sites, food source and shelter. In recent study Dagnaw & Mesele37 in Zengo Forest also reported that, the highest diversity of bird species was observed at the forest habitat. Furthermore, Eshetu et al.38 had also reported that the highest abundance of birds was recorded in dense and ticket forest habitat types. But the lowest abundance was recorded in farmland habitat types. These might be related with the fact that forest habitats are much conducive for birds in the availability of food and roosting sites.
In contrary to this study, Zerihun et al.39 stated that Wondo Genet Forest, natural forest which is dominated by few tree species is not suitable to different bird species, because it does not fulfill the feeding preferences of most birds together with risk of predation and it is preferred by few forest specialist bird species alone as they obtained sufficient cover and food necessities. The second highest bird individuals were recorded at the woodland habitat in present study area. In line with this study, Gloria30 reported that bird species diversity was higher in forest followed by woodland and shrub land than in the settlement and farmland. This might be differences in resource availability between habitats such as breeding sites, nesting material, cover, food and water restrict some species to certain habitat type while allowing others to be widely distributed.
The small number of bird species was recorded from the farmland habitat. This might be due to habitat modification for agricultural activities, road construction, removing of nesting grounds, spraying of herbicides on agricultural areas, transmitted diseases and computation of birds with domestic animals. Aggeliki et al.40 reported that farmland biodiversity continues to decline mainly because of agricultural intensification and land abandonment.
Furthermore, Anteneh et al.41 reported that permanent removal of forest components in farmland destroys species foraging and nesting resources and unnaturally enhances exposure to natural enemies such as predators and also destabilizes the balance of processes such as interspecific competition and co-existence. In contrary to this Henderson et al.42 found out that land use change is a factor in both declining and increasing of bird populations. For instance, the introduction of set-aside has resulted in a relatively large area of farmland being converted in to a habitat that is preferred by a number of farmland bird species.
In the case of species similarity, communities showed the highest similarity during the wet season than dry. The highest species similarity was observed between forest and woodland in wet season. This might be related to the availability of insects, flower composition, high canopy cover and presence of fruit. In addition to this they occur in similar locality could be occupied by similar bird species and generalist species interchanging between the two habitat types. In agreement with the findings of Kamal et al.43 avian species diversity and richness and similarity were positively correlated with the similarity in vegetation type and foliage height.
In present study high species evenness was observed at the woodland habitat. It might be related to habitat homogeneity. Species evenness might be closely linked to habitat complexity or heterogeneity; greater heterogeneity might explain the relatively low species evenness.44 The lowest evenness was recorded from the farmland. This is supported by Wilsey & Stirling45 that the increase competition particularly can result in dominant species having greater proportional abundances leading to decreased evenness.
Large number of forest bird species were found to be ranked under uncommon based on their relative abundance scores during the wet season. This might be related to vegetation complexity, inconspicuousness of small birds, roosting and feeding. Similarly, Shimelis & Afework46 stated that the presence of high number of uncommon bird species in Zegie Peninsula could be related to cutting of trees, clearing vegetation for coffee plantation and firewood production to sell to the nearest town; those activities could reduce the individual bird species. Likewise, Kalkidan & Afework26 reported that the relative abundance of birds in the forest habitat showed large number of bird species grouped as uncommon. This might be due to the vegetation complexity and inconspicuousness of small birds. In recent study Seyoum et al.28 also reported that the majority of 74% of birds were registered under uncommon species. This might be due to majority of the species had low population sizes as a result they were grouped under uncommon species. Only five bird species Swainson's Sparrow, Little Weaver, Brown-rumped Seadeater, Ruppell's Robin-Chat, Abyssinian Oriole are grouped under common species because they had relatively a greater number of individuals.
In the study period rare species was not observed, this might be the area less comfortable for the specialist bird species and the absence of rarity was the area support birds and related to the habitat condition.
The present study documented base line information about avifauna in Menagesha Amba Mariam and Gara Medhanialem forest. The occurrence of 112 species of birds confirms the importance of Menagesha forest for biodiversity protection. The study area, in spite of small in size it supports endemic, migratory and near endemic species where they get better protection and good access for foraging and nesting opportunities. Habitat type determines bird species diversity, abundance, evenness, similarity and richness. Birds are associated with different habitat types where basic requirements like nesting grounds, roosting sites water and food resources are available.
In both dry and wet seasons, the highest species diversity richness and individual abundance was recorded from the forest habitats, and the highest evenness recorded at the woodland habitat. This indicates that forest and woodland habitats are suitable for birds by providing cover nesting materials and feeding requirements. Bird diversity, abundance and evenness were high in wet season than dry seasons. The small number of bird species diversity, individual abundance and evenness were recorded from the farmland habitat. In the farmland habitat there are different human activities practiced that affect bird’s diversity and abundance, such as farming, livestock grazing, cutting of trees for charcoal, road construction, erosion and need for additional farming area.
The author would like to thank everyone who helped him to write his thesis successfully and also thanks Dr. Shimelis Aynalem and Dr. Nega Tassie for their professional guidance, intellectual stimulation, which made my study successful. Besides, I would like to thank Dr. Mezgebu Ashagrie for his support in statistical analysis and Belayneh Abebe for providing me a bird guide book for the field data collection. In addition to these thanks the local communities support me during data collection and sharing ideas. Finally, Special thanks go to his parents for their financial support.
The author declares that there is no conflict of interest.
None.

©2020 Desalegn, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.