International Journal of
eISSN: 2574-9862


Research Article Volume 7 Issue 2
1PhD student in Zoology at the Federal University of Paraná (UFPR), Laboratory of Vertebrate Biology (LABBEV), UFPR, Curitiba, Brazil. Associated Researcher at Cananéia Research Institute (IpeC), Cananéia, Brazil
2Researchers in bioacoustics and spectrographic analysis of vocalizations
3Founder and Researcher at Academo (academo.org), London, United Kingdom
4Associate Researcher at the Sustainability and Innovation Research Group (NEO), RGD/CNPq. Associate Professor of the Federal University of Goiás (UFG), Goiânia, GO, Brazil
5Amateur ornithologists, Brazil
Correspondence: Ellen F Freitas, PhD student in Zoology at the Federal University of Paraná (UFPR). Laboratory of Vertebrate Biology (LABBEV), UFPR, Curitiba, PR, Brazil, Tel +5562985803509
Received: June 15, 2023 | Published: July 14, 2023
Citation: Freitas EF, Ball E, Fernandes FF, et al. Behaviour of native birds and analysis of vocalizations, in a small reforested urban space near downtown Goiânia, Goiás state, Brazil. Int J Avian & Wildlife Biol. 2023;7(2):69-79. DOI: 10.15406/ijawb.2023.07.00193
Due to habitat loss, several bird species that are dependent on forest environments seek refuge in green spaces within cities. The objective this study was to verify if the afforestation with autochthonous fruit trees of a small urban space could provide enough resources as food, shelter, and substrate for nesting of autochthonous bird species. Between June 2020-May 2021, during the covid-19 pandemic we observed the activity of bird species that visited a small forested urban space in Goiânia, Brazil. We recorded the behavioural activities of the birds using the focal animal technique, recordings of vocalizations and photographic and video recordings. About thirty species of birds were seen visiting our small urban wooded space. The most conspicuous species were the Amazonian Motmot, Picazuro Pigeon, Ferruginous Pygmy-Owl, Rufous-Browed Peppershrike, Rufous-bellied Thrush, Yellow-chevroned Parakeet, Great Kiskadee, Blue and Yellow Macaws, and the Common Potoo. All these species rested, vocalized and took refuge in the small urban wooded space. We also observed some species that nested, and many others that fed on tree fruits, insects and earthworms. We verified the presence of Amazonian Motmots (Momotus momota) adapted to this environment when we saw adult and juvenile Motmots and found an active nest. We also verified the reproductive adaptation of the Picazuro Pigeon (Patagioenas picazuro) observing the courtship and mating behaviour and found an occupied nest, which corroborates with the reproductive adaptation of the Picazuro Pigeon. We emphasize that native fruit trees are a fundamental part of urban environments, as they guarantee food for frugivorous birds, whereas mature leafy trees provide conditions for nesting and allow birds to congregate and communicate.
Keywords: sustainable cities and communities, urban afforestation, synurbization, native birds, spectrographic analysis of vocalizations, native fruit trees, Cerrado biome
The urban areas contain small subsets of native bird species inhabiting surrounding rural areas. However, urban areas that hold tree-rich green spaces can attract several species of native birds, either through the availability of food, shelter, or nesting substrates.1,2 This process, known as synurbization, occurs when wild birds find alternative habitats appropriate for survival.1 Recently, several authors found that large urban greenspaces positively influence bird diversity in cities.3–9 However, it still remains for us to know whether the reforestation of small urban spaces available in large cities would be a sustainable and effective measure to significantly support the environmentally sustainable development of cities and, more specifically, the preservation of native flora and avifauna. These data and this doubt stimulate studies to verify more clearly how wild birds in synurbization would respond to small urban spaces reforested with species native to local biomes. Here, we describe how native birds responded to a small urban space that we reforested, mainly with fruit trees native from the Cerrado biome (also known as the Neotropical Cerrado or Tropical Savannah of South America), located near the historic centre of the capital of the state of Goiás, Brazil.
Specifically, we aimed to qualitatively assess how native birds used this small green space, how they behaved throughout the day, and whether there was reproductive adaptation of any species. The relevance of our study resides in the fact that we believe that encouraging the formation of several small urban green spaces with native fruit trees could also contribute significantly to sustain communities of native bird species in synurbization, within extensive and inhospitable urban matrices.
We conducted our study at a site 1.53 miles from the “Dr. Pedro Ludovico Teixeira Square”, adjacent to the historic administrative centre of Goiânia, the capital city of the state of Goiás, located in Midwest region of Brazil (Figure 1). According to estimates by the Brazilian Institute of Geography and Statistics,10,11 Goiânia, which has about 729 km2 of territory, in 2019 was considered the fifth largest city in Brazil in urbanized areas (≈ 301 km2), and, in 2020, the tenth most populous (≈ 1555 million inhabitants). The study area consists of three urban lots, totalling around 1700m2. While we conducted the study, one of these lots was still a vacant lot, which contained abundant guinea grass (Panicum maximum); an allochthonous herb) and a large leafy cambará (Gochnatia polymorpha), a native species proper of the Cerrado biome (Figure 1A); a central lot that contains a small residence at the back, and two medium-sized specimens of cagaita (Stenocalyx dysentericus), a species also native to the Cerrado biome (Figure 1C); and a lot used as a rustically adapted parking lot, which contained two trees, including a large acacia (Acacia spp.), and was partially covered with grasses, including the allochthonous herb signal grass (Brachiaria decumbens). Our study site is within an area with several residences and companies, with all streets and avenues asphalted. About ten years ago (more intensively about seven years ago), we reforested the central lot by planting different fruit species, mainly from the Cerrado biome (Figure 2). At the back of the central lot, we delimit an area of approximately 120 m2 (8 x 15 m) by means of a 1 m high fence aiming to protect nests of birds and chicks. We conducted our study during the preventive social isolation of 2020 and 2021 due to the covid-19 pandemic. Between June 2020 and May 2021, we registered the following bird behaviours: (i) feeding, (ii) courtship, copulation, and nesting, and (iii) vocal communication. We considered these behaviours qualitative indicators of acceptance of the small reforested urban space. Namely, if a bird fed, reproduced, or vocally communicated there, we supposed that the bird accepted well that green space. We made additional observations at the study site from September to December 2021 and in April 2022. We registered the bird activities both during the day and night. When the birds were visible or audible, we filmed them with a high-resolution video camera (Canon EOS/Rebel SL3) or recorded their voices using a portable high-definition digital recorder (Tascam Dr-05x) adjusted at 96 kHz/16 bits. We analysed the vocalizations of some species using the Spectrograph program (a project between Edward Ball at Academo.org, UK, and Prof. Michael J. Ruiz at UNC Asheville, USA). For visualizing the sound, we elaborate spectrograms (i.e., signal amplitude graphs) after eliminating the excessive environmental noise.12We identified bird species based on morphological traits and vocalizations.13–20
We identified thirty species of birds visiting our study site (Figures. 2˗4, Table 1). Other wild animals that arrived at the site included frugivorous bats, rodents, geckos, amphibians, insects, snails, slugs, and earthworms (Figure 2). We frequently observed frugivorous birds feeding on fruits of guavas (Psidium guajava), mangos (Mangifera indica), papayas (Carica papaya), murici (Byrsonima crassifolia), and ata (Annona squamosa) (Figure 2, Video 1 in Supplementary Material). We saw the Rufous-bellied Thrushes (Turdus rufiventris), Rufous Horneros (Furnarius rufus), and Chalk-browed Mockingbirds (Mimus saturninus) feeding more often on murici fruits (Figure 2I). Below, we detail the behaviour of those more conspicuous species in the study site.

Figure 1 Tree species remaining from the Cerrado biome and planting of fruit trees in a small reforested urban space near downtown Goiânia, Goiás state, Brazil. English common name follows the Portuguese common name. (A) Cambará (Gochnatia polymorpha). (B) Cagaiteira, cagaita (Stenocalyx dysentericus). (C) Muricizeiro, nance (Byrsonima crassifolia). (D) Araticunzeiro, marolo (Annona crassiflora). (E) Mangabeira, mangaba rubber tree (Hancornia speciosa). (F) Cajá-anão, ambarella (Spondias dulcis; dwarf variety). (G) Pequizeiro, pequi (Caryocar brasiliense, dwarf variety). (H) Cajueiro, cashew (Anacardium occidentale). (I) Jaboticabeira, Brazilian grape (Plinia cauliflora). (J) Guarirobeira, guariroba (Syagrus oleracea). (K) Gravioleira, soursop (Annona muricata). (L) Limoeiro Galego, Galician lemon (Citrus aurantifolia). (M) Ata, sugar apple (Annona squamosa). (N) Lichia, lychee (Litchi chinensis). (O) Goiabeira, guava (Psidium guajava). (P) Macaubeira, grugru (Acrocomia aculeata). (Q) Videira, grape (Vitis labrusca). (R) Seriguela, purple mombin (Spondias purpurea). (S) Bananeira, banana (Musa sp.)

Figure 2 The food resources available for birds and insects and plant-animal interactions in a small reforested urban space near downtown Goiânia, Goiás state, Brazil Observations came from a study conducted between June 2020 and May 2021, during the covid-19 pandemic. (A) Pequizeiro flower. (B) Pequi fruit (Caryocar spp). (C) Cagaitas (Stenocalyx dysentericus). (D) A hoverfly (Syrphidae) pollinating ambarella (Spondias dulcis) flowers. (E) Nymphs and adults of leafhoppers (Aethalion reticulatum) sucking phloem sap from twigs of mango (Mangifera indica) and arapuás or dog-bees (Trigona spinipes) licking the honeydew produced by leafhoppers. (F) Caterpillar. (G) Papaya with fruit partially eaten by an Amazonian Motmot pair, which rests on a manioc branch and cashew near its nest. (H) Papaya fruit consumed by birds. (I) Fruits of the Muricizeiro or nance (Byrsonima crassifolia). (J) Acerola cherry (Malpighia emarginata). (K) Grape (Vitis labrusca). (L) Cashew (Anacardium occidentale) fruit. (M) Banana (Musa sp.) bunch. (N) Ata or sugar apple (Annona squamosa) fruit. (O) Cajá-anão, ambarella (Spondias dulcis) fruit. (P) Sabina mango (Mangifera indica) fruit. (Q) Macaubeira (Acrocomia aculeata) with flowering bunch, and later, (R) with coconuts (S). (T) Mango and ata fruit consumed by birds.

Figure 3 The activity of Amazonian Motmots (Momotus momota) during 2020-2022 in a small reforested urban space near downtown Goiânia, Goiás state, Brazil. (l) Photographic records taken between June 2020 and September 2021, during the covid-19 pandemic. (A-C) An Amazonian Motmot eats an earthworm next to two Picazuro Pigeons (Patagioenas picazuro). (D) An Amazonian Motmot pair rests in the cashew treetop over their nest. (E-F) Adult individuals rest on branches of papaya (Carica papaya) and murici (Byrsonima crassifolia), respectively. (G) An adult individual perched on a hoe handle in front of the entrance of its nest (arrow), excavated in an artificial ravine (Finding date: 25 September 2021). Under the nest entrance, dogs dug a hole, attempting unsuccessfully to prey upon adults or nestlings (arrowhead). (H)- (K) An Amazonian Motmot enters its nest. (L) Tunnel entrance leading to the nest. The nest was in an underground chamber at the bottom of the tunnel both pair members excavate the tunnel. (M-P) A young Amazonian motmot calmly lands on the floor in front of the entrance of a residence, eats a small insect and flies close to the ground, showing tolerant to humans. (Q) A Ferruginous Pygmy-Owl (Glaucidium brasilianum) perched on a tree branch.

Figure 4 Some of the bird species observed during 2020-2021 in a small reforested urban space near downtown Goiânia, Goiás state, Brazil. Photographic records taken between June 2020 and April 2022. (A-B) White-eyed Parakeets (Psittacara leucophthalmus) on guava. (C) A Yellow-chevroned Parakeet (Brotogeris chiriri) vocalize from a mango (Mangifera indica) treetop. (D-F) Squirrel cuckoos (Piaya cayana) perched on the treetop of papaya, cambará, and condessa (Annona reticulata), respectively. (G) A female Great Kiskadee (Pitangus sulphuratus) feeds her fledgling. (H) A Swallow-tailed hummingbird (Eupetomena macroura) on a break on the foliage. (I-J) A Rufous Hornero (Furnarius rufus) on the ground. (K) Female Ruddy Ground-dove (Columbina squammata) incubates in her nest built in a Galician lemon tree (Citrus aurantifolia). (L) A juvenile Ruddy Ground-dove (Columbina talpacoti) rests on the guariroba (Syagrus oleracea) foliage after practice flight. (M) A Saffron finch pair (Sicalis flaveola) reacts to their reflection in a car's rear-view mirror. (N) A Blue and Yellow Macaw (Ara ararauna) rests on a street-lighting lamp, and (O-P) flies from a cambará (Gochnatia polymorpha). (Q) An Amazonian Motmot feeds on leftover industrialized dog food. (R) A Common Potoo (Nyctibius griseus) rests on a tree branch during the day, and (S) vocalizes on a wooden pole on the night.
|
English common name |
|
Portuguese common name |
Scientific name |
Order and Family |
Frequency of sightings* |
|
Picazuro Pigeon |
|
Asa-branca |
Patagioenas picazuro |
Columbiformes: Columbidae |
VF |
|
Great Kiskadee |
|
Bem-te-vi |
Pitangus sulphuratus |
Passeriformes: Tyrannidae |
VF |
|
House Sparrow |
|
Pardal |
Passer domesticus |
Passeriformes: Passeridae |
VF |
|
Ruddy Ground-dove |
|
Rolinha-caldo-de-feijão |
Columbina talpacoti |
Columbiformes: Columbidae |
VF |
|
Rufous Hornero |
|
João-de-barro |
Furnarius rufus |
Tyranni: Furnarioidea |
VF |
|
Amazonian Motmot |
|
Martim-pescador-de-mata-virgem, Udu-de-coroa-azul
|
Momotus momota |
Coraciiformes: Momotidae |
VF |
|
Yellow-chevroned Parakeet |
|
Periquito-de-encontro-amarelo |
Brotogeris chiriri |
Psittaciformes: Psittacidae |
F |
|
White-eyed Parakeet |
|
Periquitão-maracanã |
Psittacara leucophthalmus |
Psittaciformes: Psittacidae |
F |
|
Ferruginous Pygmy-Owl |
|
Caburé |
Glaucidium brasilianum |
Strigiformes: Strigidae |
MF |
|
Blue-black Grassquit |
|
Tiziu |
Volatinia jacarina |
Passer: Thraupidae |
MF |
|
Rufous-browed Peppershrike |
|
Pitiguari |
Cyclarhis gujanensis |
Passeriformes: Vireonidae |
LF |
|
Smooth-billed Ani |
|
Anu-preto |
Crotophaga ani |
Cuculiformes: Cuculidae |
LF |
|
Scaled Dove |
|
Rolinha-fogo-apagou |
Columbina squammata |
Columbiformes: Columbidae |
LF |
|
Saffron Finch |
|
Canário-da-terra |
Sicalis flaveola |
Passeriformes: Thraupidae |
LF |
|
White-tipped Dove |
|
Juriti |
Leptotila verreauxi |
Columbiformes: Columbidae |
LF |
|
Rufous-bellied Thrush |
|
Sabiá-laranjeira |
Turdus rufiventris |
Passeriformes:Turdidae |
LF |
|
Swallow-tailed Hummingbird |
|
Beija-flor-tesoura |
Eupetomena macroura |
Apodiformes: Trochilidae |
R |
|
Tropical Kingbird |
|
Suiriri |
Tyrannus melancholicus |
Passeriformes: Tyrannidae |
R |
|
Chalk-browed Mockingbird |
|
Sabiá-do-campo |
Mimus saturninus |
Passeriformes: Mimidae |
R |
|
Shiny Cowbird |
|
Chupim |
Molothrus bonariensis |
Passeriformes: Icteridae |
R |
|
Green-barred Woodpecker |
|
Pica-pau-carijó |
Colaptes melanochloros |
Piciformes: Picidae |
R |
|
Squirrel Cuckoo |
|
Alma-de-gato |
Piaya cayana |
Cuculiformes: Cuculidae |
R |
|
Black-fronted Nunbird |
|
Chora-chuva-preto |
Monasa nigrifrons |
Galbuliformes: Bucconidae |
R |
|
Common Potoo |
|
Urutau |
Nyctibius griseus |
Nyctibiiformes: Nyctibiidae |
R |
|
Green-winged Saltator |
|
Trinca-ferro |
Saltator similis |
Passeriformes: Thraupidae |
R |
|
Dubois's Seedeater |
|
Papa-capim-costas-cinza |
Sporophila ardesiaca |
Passeriformes: Thraupidae |
R |
|
Purple-throated Euphonia |
|
Fim-fim |
Euphonia chlorotica |
Passeriformes: Fringillidae |
R |
|
Buff-necked Ibis |
|
Curicaca |
Theristicus caudatus |
Pelecaniformes: Threskiornithidae |
R |
|
Blue-and-yellow Macaw |
|
Arara-canindé |
Ara ararauna |
Psittaciformes: Psittacidae |
R |
|
Roadside Hawk |
|
Gavião-carijó |
Rupornis magnirostris |
Accipitriformes: Accipitridae |
R |
Table 1 List of bird species identified between June 2020 and May 2021 in a small reforested urban area near downtown Goiânia, Goiás state, Brazil
*The order of species in the list is according to the frequency of sightings, in descending order: VF, very frequent (100–80% of sightings); F, frequent (79–50% of sightings); MF, medium frequent ( 49–30% of sightings); LF, low frequent (29–10% sightings); R, rare (< 10% sightings).
Amazonian Motmots (Momotus momota) visited the forested space more often after rainy dawns or nights, especially after residents raked the leaves off the ground early in the morning (06:00˗08:00 h). They also visited the forested space several times in the afternoon, mainly between 16:00 and 18:00 h, preferably after the rains. During this period, we observed young and adult motmots perching more often on fruit trees than on the ground. The trees where they perched were guava, mango, murici, cashew, and papaya. Less often, we observed a Motmot pair resting as early as 02:30 in the cashew or papaya, close to the nest (Figures 2 and 3, Video 2˗5 in Supplementary Material).
The Amazonian Motmots fed on papaya, earthworms, and insects of varying sizes and stages (e.g., small beetles, caterpillars, and adult lepidopterans). Occasionally, they fed on leftover processed dog food (Figure 2 and 4Q, Videos 3 and 4). Sometimes, they beat the food in the dog feeder to break it into smaller pieces that were easier to swallow. The motmot pair fed on papaya fruit or insects attracted by the intact or already pecked fruits (Figure 2, see also Video 2). We observed an adult motmot perched on a lower branch of cashew (planted next to a ravine at the back of the studied area) emitting high-pitched and repetitive voices, seemingly because one of the dogs stared at her from outside the isolated site, ≈ 5 m from the bird (Video 5). As we inspected that area, we were as close as 3 m from the adult motmot. Immediately, he emitted louder and more intensely high-pitched and repetitive hoots. We interpreted it as an alarm call. Simultaneously, he flew restlessly from tree to tree and on a nearby wall. We supposed those responses reflected the protective behaviour of its nest against predators or intruders. After this, we carefully examined the area and found an Amazonian Motmot nest. As the motmot realized we were not a threat, he calmed and stopped chirping. Promptly, he landed on a hoe handle leaning close to the nest entrance (Figure 3G). Then he flew, landed, and quickly entered the nest (Figure 3G-L, see also Video 6 in Supplementary Material).
The nest was in a ravine, located about 1.5 m from the back wall of the central lot, under the shade of papaya and cashew (Figure 3, Video 6). This ravine resulted from an earth cutting done approximately ten years ago to build a retaining wall along the back extension of the central lot of the studied area. Finally, workers did not construct the wall nor return the earth to the excavation, leaving an artificial ravine like a natural one. The shape of the tunnel entrance excavated underground by motmots was like a rudimentary convex pentagon (Figure 3L). Each of the two top edges and two lateral edges were ≈ 4.5 cm and ≈ 7.5 cm in length, respectively. The lower edge was ≈ 8.5 cm in length. The entrance shape also resembled the facade of a house with a roof. The union of the top edges was 6 cm from the top of the artificial ravine, and the lower edge was ≈ 1 m above the ground. The shape of the upper part of the tunnel entrance could result from the constant wear caused by the motmot's long tail when entering and leaving the nest (Figure 3K-L). Because this was the only Amazonian Motmot pair in the area, we did not intervene much more in the nest. An eventual inspection of its interior could have caused this pair to abandon its nest and, consequently, to lose their offspring. We also were aware that descriptions of nests of this species are available in the scientific literature.18–21 Amazonian Motmots also responded protectively when other birds approached the nest. On 17 December 2021, at 14:30 h, when a motmot pair rested in the cashew treetop near the nest, a Ruddy Ground-Dove landed close. Immediately, one of the pair members repelled her with a strong peck. After a few minutes, the Ruddy Ground-Dove returned and landed on the ground. Then, one of the motmots flew against it and chased her away (14:49 h). Soon after, the motmot entered its nest. After ≈ 3 min, the other motmot left the treetop and landed on a fallen branch near the nest entrance. At 16:29 h, one of the motmots returned to the cashew treetop and remained here until 16:38 h, and he returned to the nest.
Amazonian Motmots vocalized more and were more visible at dawn (06:00˗07:00 h) when searching for food on the ground. We recorded their vocalizations on 5 January 2021 at 06:34 h. First heard at around 0:02 s (yellow arrow in Figure 5), Amazonian Motmots repeated vocalizations at 0:05.5 s (different from the one described above – see yellow arrow in Figure 5). Motmots issued single hoots, each lasting about 0.3 s and having a fundamental frequency of about 400 Hz. At 0:09 s in the video, the single hoots became a series of 11 short pulses for around 0.6 s. The fundamental frequency decreased slightly while the motmots vocalized. Motmots repeated this last vocalization 2 s later, at some higher fundamental frequency (450 Hz). In this latter vocalization, we detected two distinct frequencies. Possibly, two individuals duetted, or one individual vocalized polyphonically. Because we recorded vocalizations at sunrise, the audio gathered the voices of other bird species. Spectrogram 1 (in Supplementary Material) begins by showing a flock of Yellow-chevroned Parakeets (Brotogeris chiriri) talking to each other. A rooster crowing is audible at 0:03 s – it starts with a fundamental frequency of 2.2 kHz and descends in the course of ≈ 1 s to 1.8 kHz. This pattern repeats at 0:23 and 0:3 s. From now and then, a bird tweets loudly (close to the microphone), resulting in an evident burst of intensity on the spectrogram (see at 0:31 and 0:39 s).
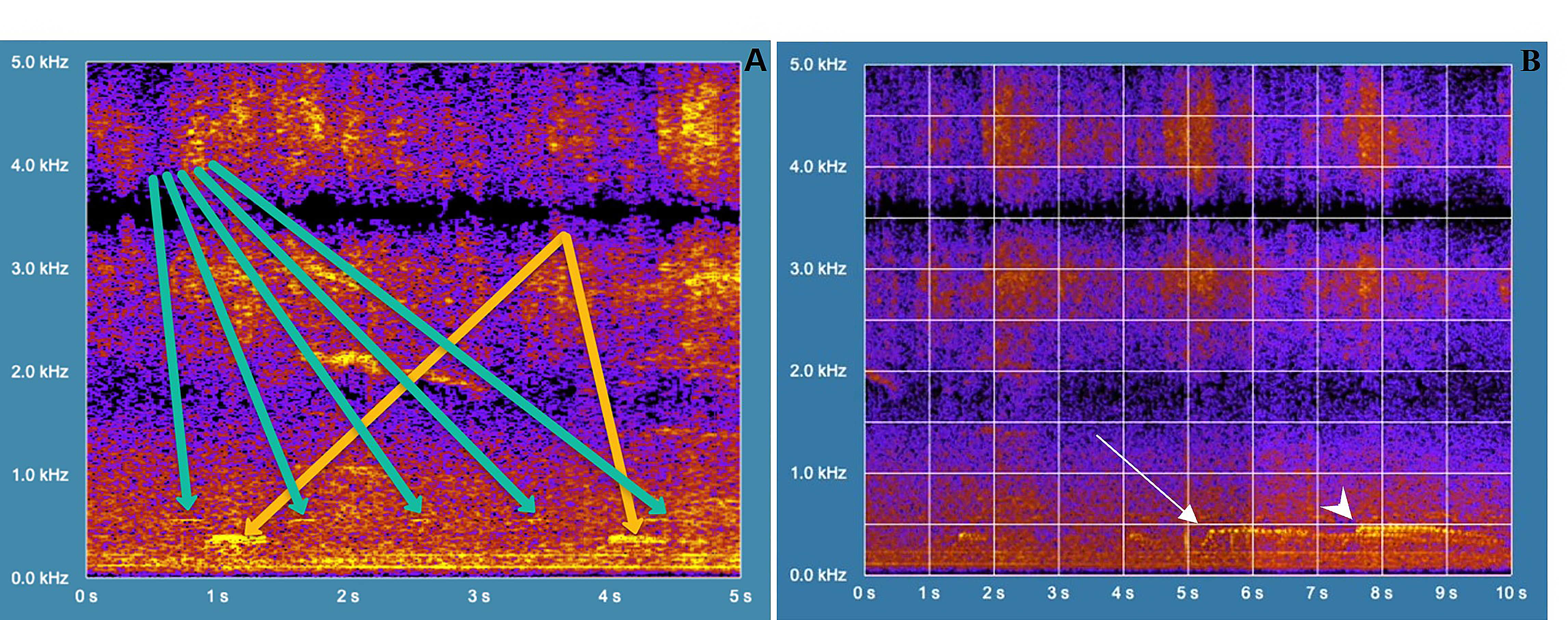
Figure 5 Spectrogram of sound emissions from Amazonian Motmot (Momotus momota) and other bird species present in a small urban reforested space near downtown Goiânia, Goiás state, Brazil. (A)Vocalizations of Yellow-chevroned Parakeet (green arrows) and Amazonian Motmot (yellow arrow). (B) Starting two distinct sound frequencies heard, which overlap (arrow and arrowhead), a duet call emitted by Amazonian Motmots.
The Picazuro Pigeon (Patagioenas picazuro) was the bird species most frequent in our study site. As soon as dawn broke, Picazuro pigeons searched for food on the ground. At sunrise (≈ 06:30 h), one or several individuals stood on the ground doing small jumps and energetically flapping to make room, demarcate their territory, or attempt to copulate. We more often saw one to three individuals, sometimes up to eight, in that activity. While feeding, Picazuro pigeons tolerated the proximity of Amazonian motmots, not showing some mutual aggression. We observed Picazuro pigeons less than 0.5 m away from an Amazonian Motmot eating an earthworm (Figure 3, Video 4). While doing our study, a Picazuro Pigeon pair nested on a mango (Mangifera indica) ≈ 6.5 m from a small residence. The nest was on a branch bifurcation ≈ 3.5 m above the ground. It was slightly flat-shaped and composed of thin ad dry twigs loosely intertwined. The nest cup contained only one egg. Residents informed us that Picazuro Pigeons usually nest in this place, and they once found an egg on the ground just below the nest. In contrast, we observed that Ruddy Ground-Dove (Columbina squammata), which is a smaller Columbidae, when compared to Patagioenas picazuro, preferred to build its nest in the Galician lemon (Citrus aurantifolia), which we also planted and grew up in the studied area (Figure 4K). We believe that this is because their many and sharp spines provide some protection against predators, such as the Ferruginous Pygmy-Owl (Glaucidium brasilianum), also observed in the studied area (Figure 3Q). Often, we observed an adult male and female Picazuro Pigeon together. Young Picazuro Pigeons and Ruddy Ground-Doves took refuge and rested on cassavas (Manihot esculenta) and the smaller trees during the first flights (Figure 4L). Sometimes, we saw flocks with approximately 12 Picazuro Pigeons, resting and observing the studied area perched in taller trees, mainly on the leafy cambará (Gochnatia polymorpha).Then the individuals watched on the ground to see if there were dogs and to decide when to go down to eat.
At the study site, we observed a Picazuro Pigeon pair in courtship behaviour (Video 7). First, the male neared the female while on a lemon branch. Then, the female lowered and raised his head and neck repeatedly in a signal she accepted him. Immediately, the male fluttered its wings and mounted the female for almost 2 s. After copulation, the male again beat his wings and lifted his head and neck over the female, who responded mutually. The female repeated the acceptance signals lowering and raising her neck and head about ten times. Again, the male mounted the female for almost 2 s. Then, the male returned to his position on the branch. After 16 s, the male mounted the female for ≈ 2 s. When the male ended copulation, he repeated the wing beating and lifted his head again over the female. However, the female flew to another branch of the same tree. Courtship and copulation lasted ≈ 53 s, with 16 to 17 s between copulas (Video 7 in Supplementary Material).
Picazuro Pigeons vocalized more when they were among the semi-open foliage of the mango treetop. They emitted a defined vocalization at a frequency of 500 Hz. At 0:01 s, we faintly heard the first Picazuro Pigeon. At 0:07˗0:10 s, the same individual issued four distinct syllables. The first syllable was a whistle of ≈ 0.8 s. This syllable started at around 450 Hz, then raised to 500 Hz, and descended at the initial frequency. About 0.3 s later were two distinct "pips" of 0.1 s each, with intervals of ≈ 0.5 s and a frequency of approximately 600 Hz. After 0.5 s, we heard the final note. Like the first note, it started at around 450 Hz, but this time raised at ≈ 550 Hz and was slightly shorter in duration (about 0.5 s). Overall, vocalizations lasted ≈ 2.5 s. At 0:18˗0:22 s, the same individual vocalized similarly but some more extended. At 0:27˗0:29 s, another individual vocalized as the first one, but the “pips” were higher in frequency (≈ 750 Hz) and slightly longer in duration. The second “pip” was somewhat lower in frequency than the first. At 0:34˗0:37 s, the first individual vocalized as it did at 0.18 s (Spectrogram 2 in Supplementary Material). The snaps from pigeons flapping during these jumps are visible in Spectrogram 1 (between 3.5 to 5 s).
We detected the Ferruginous Pygmy-Owl (Glaucidium brasilianum) more frequently by its vocalizations (Spectrogram 3 in Supplementary Material) which we heard during the day (Figure 3Q), early morning, and mainly during the night. On one occasion at dusk, we observed this owl species near the Picazuro Pigeon nest among the foliage of a mango treetop. Sometimes, Ferruginous Pygmy-Owl perched on the branches of other trees or the wall limiting the study area. At sunrise, we also saw a Ferruginous Pygmy-Owl on a wall, hidden among a muricizeiro’s foliage, keeping at a grey rat moving on the branches. The rat remained motionless in an attempt not to be noticed by the owl. When we tried to get close to film the eventual prey capturing, we inadvertently scared the rat away, which fled very quickly, running on the branches from one tree to another, going down the ground, and finally hiding among the dense leaf-litter layer.
Ferruginous Pygmy-Owls vocalized on a rainy dawn, at 04:14 h. The first vocalization consisted of 13˗15 short whistles repeated at varying intervals (Spectrogram 3). At 0:02 s in the spectrogram video, an individual issued 15 distinct “chirps” (i.e., short whistles rapidly increasing in pitch) at almost three chirps per second. Each chirp lasted ≈ 0.1 s. Thus, the duration of this vocalization was ≈ 5 s. As vocalization progressed, chirps at the end were somewhat longer than at the start. Subsequently, we observed that the second and third vocalizations consisted of 13 chirps each. The second vocalization started at 0:25 s and the third at 0:58 s. The Ferruginous Pygmy-Owl issued notes in low volume, with each chirp beginning at a frequency ≈ 500 Hz and rapidly ascending to ≈ 2 kHz. As the vocalization went on, this range gradually decreased. The ending chirps had a smaller range starting at nearly 1.5 kHz and finalizing near 1.8 kHz. The same pattern occurred at 0:25 s and 0:58 s.
The Rufous-Browed Peppershrikes (Cyclarhis gujanensis) at our study site showed discreet behaviour. We detected them only by their beautiful songs. Sometimes, we saw them among the foliage, higher up on mango, while singing. Peppershrikes issued “warbles” near 2.5 kHz, each near 0.5 s (Spectrogram 4 in Supplementary Material). The first occurred at 0:03 s of recording, and again at 0:10 s, 0:14 s, 0:19 s, and beyond, repeating them approximately every 5 s. At 47.5 s, we detected a fascinating descending, frequency-modulated vocalization. This vocalization started at ≈ 3 kHz and descended to ≈ 2 kHz over 3.1 s. All of that was modulated by a wave somewhat sine of an amplitude near 0.3 kHz. We observed slight discontinuities at each peak in this signal. Throughout this part of the call, there were five distinct complete frequency-modulation periods. Based on that information, we calculate the modulation period as 0.62 s (3.1 s/5 s) and the frequency as 1.6 Hz (1/0.62). We heard a call similar to this at 1:05 and 1:19 in the video. However, while the first two times we hear that call, the overall frequency goes down, the third and last time, the frequency starts low, increases and then goes down again. Finally, the video ends with a recurrence of the tweets described above.
We recorded a Rufous-bellied Thrush pair while it was on the ground eating fallen murici fruits. When we approached, the female flew and landed on a nearby tree. Following, we describe the duet call of that Rufous-bellied Thrush pair (Spectrogram 5 in Supplementary Material). At 0:01 s, one of the thrushes called the other. Quickly, the other responded at 0:02 s. At 0:04 s, the first again vocalized, and at 0:05 s, the second one responded. They continued duetting up to 0:10 s when the audio ended. Each call in the sequence began with a very short “squawk” of approximately 0.1 s and between 2500 and 3000 Hz. Then, each thrush quickly emitted a defined whistle that started at a fundamental frequency near 1250 Hz and raised to around 1750 Hz over approximately one-third of a second.
In Spectrogram 1, we detected the voices of the yellow-chevroned Parakeet (Brotogeris chiriri) flock (Figure 4C). At 0:02 s, parakeets emitted soft “hoots” approximately every 0.9 s at a fundamental frequency of almost 550 Hz (see green arrows in Figure 5A). Each hoot lasted ≈ 0.1. Those hoots were hard to detect on the spectrogram due to their low intensity. The lower note was ≈ 500 Hz, so the gridline masked it on the spectrogram. Throughout Spectrogram 2, we could also hear the chatter from yellow-chevroned Parakeets over a broad frequency range above 2500 Hz. Finally, at 0:58sec do Spectrogram 4 we can also hear the hoots of a yellow-chevroned Parakeet, contiguous to the beginning of one of the C. gujanensi calls.
Toward the end of Spectrogram 1, we also heard the squawking of a Great Kiskadee (Pitangus sulphuratus). Over Spectrograms 1, 2, 4, and 5, and Video 3 and 4, we also hear the vocalization of House Sparrows as part of the background noise.
Between June and July 2020, Blue and Yellow macaws (Ara ararauna) flew daily over the study site. On 16 April 2021, at 10:00 h and 14:40 h, we observed several individuals in the cambará branches. Three individuals vocalized on a mature cambará for ≈ 9 min in the afternoon (Figure 4N-P). On 18 and 19 April 2021, at 10:00 and 12:30 h respectively, several Blue and Yellow Macaws were on the branches of a cambará. On 28 April, at 16:30 h, we found a Blue and Yellow Macaw’s nest on the trunk of a dead Imperial palm (Roystonea oleracea) in the backyard of a house very close to the study site (Video 8 in Supplementary Material).
On 23 October 2021, during the night, a Common Potoo (Nyctibius griseus), also known as “Urutau”, in Brazil, vocalized on a wooden pole within our study site (Figure 4S). We observed it for three consecutive nights, not returning the following days. On 9 April 2022, during the day, a Common Potoo rested without vocalizing on a tree branch (Figure 4 R-S, see also Audio 1 in Supplementary Material).
The presence of native birds in the small reforested urban space we studied was consistent with a synurbization process.1 Our evidence revealed that our reforested site constituted an efficacious refuge for native birds. The adjustment of several native bird species to the small urban greenspace was evident as they fed, mated, nested, and vocalized naturally in the place. Among our findings, the most notable were the nesting of the Amazonian Motmot and Pigeon Picazuro, and the observation of the courtship behaviour of this species, in this peri-domiciliary environment, reforested, but very near to the center of a large city. Our observations of the breeding behaviour of these species, among others, reflected that the birds adapted quite well to our small greenspace. We did not find in the scientific literature reports of the Amazonian Motmot nesting nor about the mating of Picazuro Pigeons in urban peri-domiciliary spaces in our metropolitan region. In the case of M. momota, the discovery of an active nest is relevant, as it is a bird that already has the status of endangered species (category – EN) in some regions of Brazil, such as the Center of Endemism of the state of Pernambuco – located in Northeast region of the country –,22 the Pantanal – in Midwest region –,23,24 and the state of Paraná – in South region –,25 due to progressive habitat destruction as urban environments and agricultural land expand.26,27 However, we still cannot say exactly that some subspecies of M. momota existing in the Cerrado biome would be endangered, since, currently, “There is no consensus regarding the validity of some subspecies of Momotus momota, requiring a taxonomic revision.” (ICMBIO, 2018, p. 220).28
The external characteristics of the Amazonian Motmot’s nest that we found in an artificial ravine were like those previously described by Skutch18 and Sick.19 These authors mention that the Amazonian Motmots pairs in their natural habitat dig a tunnel, from 60 cm to 2 m (generally just over 1 m long), ending in an underground chamber, where the female lays about three eggs. We believe the similarity between the artificial ravine and a natural ravine pleased and encouraged the motmot pair to build their nest there. Amazonian Motmot pairs commonly build their nests in natural walls (ravines) along riparian forests,18–20,29–31 many of which are often devastated by increasing deforestation.15,26,27 To avoid the Amazonian Motmot pair abandoning the reforested space, we decided not to dismantle the nest only for measurement proposes.
On two occasions, dogs accidentally entered the nesting site and excavated under the nest entrance, seemingly attempting to prey on the female or her offspring. To prevent the dogs destroyed the nest, we covered the hole with the earth, using a hoe. We also observed dogs killing birds, an adult House Sparrow (caught when it flew into the foliage on the ground), a juvenile Great Kiskadee, and a juvenile Pigeon Picazuro (captured during the first flights). These events raise the need to prevent the free displacement of dogs to urban greenspaces where birds nest under the ground or in trees and young birds start learning to fly. The impacts of dogs on ground-nesting birds include the stress of fledglings when they are outside their nests for long periods, egg crushing, predation on chicks and adults, and partial or total nest destruction. Those can cause devastating impacts on populations of threatened species.32,33
The Picazuro Pigeon is a clear example of successful synurbization in Brazil. In Midwest Brazil, three to four decades ago, this species was present almost exclusively in wild and rural areas. Picazuro Pigeons assiduously visited croplands to feed on rice and corn grains. However, many individuals died after ingesting seeds with herbicide residues. The use of herbicides is a common agricultural practice in Brazil. In addition, Picazuro Pigeons became extremely skittish at that time due to the constant persecution by hunters. When Picazuro Pigeons left their nesting sites to visit croplands, they habitually stood in tall, isolated trees to warn about the presence of humans. Often, Picazuro Pigeons stood on canela-de-velho (Aspidosperma brasiliense), a widely distributed in the Cerrado biome.34 Currently, Picazuro Pigeons are more confident of humans, visiting urban greenspaces such as squares with lawns, wooded lots, and parks, where they are at less predation risk and can feed more peacefully.35
The variety of native fruit trees attracted several native bird species to our small reforested urban space. Yellow-chevroned Parakeets and White-eyed Parakeets ate daily mainly guava and mango fruit. On three different days, White-eyed Parakeets also pecked at muricizeiro fruits. In general, several species fed on fruits from several tree species. Thus, we presumed that those species are generalist fruit-feeders. We observed a more frequent consumption of murici fruits by Rufous-bellied Thrushes, Rufous Horneros, and Chalk-browed Mockingbirds, which preferred to peck the ripest fruits, fallen to the ground, perhaps due to their more delicate beaks.
Possibly, bird congregation in our small urban greenspace attracted the Ferruginous Pygmy-Owl. This owl species often prey upon small and medium-sized birds. In addition to birds, it also feeds on insects and small vertebrates, such as lizards and mice.17,19,36 All these prey types were available in our small reforested urban space. The behaviour of the Rufous-browed Peppershrike in our small reforested urban space was consistent with that previously described in the literature. This species typically inhabits open habitats or forest edges. Individuals inhabiting forests usually rest at the treetop and forage through foliage and branches. Often, they go unnoticed in the vegetation but are perceptible by their melodious songs.37,38 Possibly the variety of foods attracted Rufous-browed Peppershrikes to our small urban greenspace. This species feeds on fruits, insects, or small vertebrates.17,38 The frequent and intensive vocalizations of several native birds in our small reforested urban space revealed that they naturally maintained vocal communication, despite the human activity in the lieu. The vocalizations of the Picazuro Pigeons resembled that of non-urban sites.19 In the case of the Rufous-Browed Peppershrike, we detected a singing with different notes and frequencies emitted by the bird. That contributes to increasing our understanding of the vocalizations of that species. According to our spectrogram analysis, a Rufous-bellied Thrush pair vocalized differently from its typical singing, presenting duet call with different and higher frequencies, during their communication. Sick19 described the singing of this species as notes of low intensity yet imposing and very evident, with intense “jokes” during its movements. On the other hand, Souza Filho et al.39 detected four different phrases with eleven distinct musical notes in the Rufous-bellied Thrush singing.
Our results, combined with those from other studies, reinforce the ecological benefits of planting native trees in urban spaces.3–9 First, native fruit trees assure food availability for frugivorous bird species, and also for other animals that feed on the sap, flowers and fruits of these trees, and consequently increase the prey availability to native predators such as pygmy owls, among others animals. Second, mature leafy trees favour the nesting of several species of birds and the survival of chicks. Third, small urban green spaces allow birds to congregate and communicate. Indirectly, the synurbization of native birds also results in engaging recreational green spaces for human, which can benefit the quality of life of cities residents. The features of native trees strongly attracted native birds to our study site. Multiple fruits and microhabitats offered by those trees were fundamental for those native birds established in the lieu. Some key features of these tree species are their resistance, perenniality, and easy cultivation. We therefore recommend that citizens and local authorities include these tree species for reforesting backyards, parks, avenues, or vacant spaces within Goiânia, among other municipalities that develop in regions of the Cerrado biome, remembering that planting different species of trees is essential for maintaining biodiversity. That also will allow rapidly expanding greenspaces, sustainably, for the benefit of native animals in synurbization and humans.
This study was conceived and undertaken with the primary objective of encouraging urban tree-planting initiatives by local communities, with a view to the environmentally sustainable development of cities, the preservation of native species of flora and fauna, as well as improving the quality of life of residents of large cities. We extend our gratitude to the Academo.org (London, UK) for their invaluable technological assistance in conducting dynamic spectrographic analysis of bird vocalizations. Additionally, we express our sincerest appreciation to the esteemed Editors and diligent Reviewers for their invaluable suggestions in preparing the article.
The authors declare that there are no conflicts of interest.
Video 1 A White-eyed Parakeet (Psittacara leucophthalmus) pair rests and feeds on guava fruits in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Video 2 Adult Amazonian Motmot (Momotus momota) rests and feeds on a murici (Byrsonima crassifolia) and cleans its beak after feeding in a small reforested urban space close to downtown Goiânia, Goiás state, Brazil.
Video 3 A juvenile Amazonian Motmot (Momotus momota) calmly lands on the floor in the front of the entrance of a residence, eats a small insect and flies close to the ground, showing tolerance of humans, in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Video 4 An Amazonian Motmot (Momotus momota) eats an earthworm near two Picazuro Pigeons (Patagioenas picazuro) in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Video 5 An Amazonian Motmot emits differentiated and repetitive vocalizations, with short intervals, as a warning call due to researchers approaching its nest in a small reforested space near downtown Goiânia, Goiás state, Brazil.
Video 6 An active nest of Amazonian Motmot (Momotus momota) in a small reforested urban area near downtown Goiânia, Goiás state, Brazil. Finding date: 25 September 2021.
Video 7 Picazuro pigeons (Patagioenas picazuro) in courtship behaviour and copulation in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Video 8 A Blue-and-yellow Macaw (Ara ararauna) pair establishes a nest on the trunk of a dead Imperial palm (Roystonea oleracea) in the backyard of a neighbouring house of the small reforested space studied, close to downtown Goiânia, Goiás state, Brazil.
Spectrogram 1 Spectrographic analysis of the Amazonian Motmot (Momotus momota) vocalizations recorded between sounds emitted by other species of birds in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Spectrogram 2 Spectrographic analysis of the Picazuro Pigeon (Patagioenas picazuro) vocalizations recorded among the sounds emitted by Yellow-chevroned Parakeet (Brotogeris chiriri) in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Spectrogram 3 Spectrographic analysis of the Ferruginous Pygmy-Owl (Glaucidium brasilianum) vocalizations recorded in a small reforested urban area close to downtown Goiânia, Goiás state, Brazil.
Spectrogram 4 Spectrographic analysis of the Rufous-browed Peppershrike (Cyclarhis gujanensis) vocalizations recorded in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Spectrogram 5 Spectrographic analysis of the Rufous-bellied Thrush (Turdus rufiventris) vocalizations recorded in a small reforested urban space near downtown Goiânia, Goiás state, Brazil.
Audio 1. The Common Potoo (Nyctibius griseus) emits its typical nocturnal chirp in small reforested urban space close to downtown Goiânia, Goiás state, Brazil.

©2023 Freitas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.