International Journal of
eISSN: 2574-9862


Research Article Volume 8 Issue 1
1Grupo de Investigación en Fauna Amazónica, Colombia
2Asociación Ornitológica del Caquetá Colciencias, Colombia
3Amerisur Exploración Colombia Limitada, Colombia
Correspondence: Carrillo Chica Esteban, Grupo de Investigación en Fauna Amazónica Colombiana. Carrera 68B No. 98A-30 apt 301, Bogotá, Colombia, Tel +57 3202322168
Received: January 31, 2024 | Published: February 14, 2024
Citation: Carrillo-Chica E, María ADH. Avifauna of an exploratory oil drilling area in a previously deforested amazonian landscape. Int J Avian & Wildlife Biol. 2024;8(1):12-18. DOI: 10.15406/ijawb.2024.08.00207
Oil exploration and exploitation activities have negative effects on biodiversity. This is mainly due to secondary causes such as increased colonization following the opening of new roads. However, little is known about the effects that oil industry may have on previously deforested landscapes. In this study, we characterized the avifauna of an exploratory oil drilling area in the Colombian Amazon, where the landscape is dominated by pastures and monoculture crops with some patches of scattered secondary forests. The avifauna was typical of intervened Amazonian landscapes in which most specialist forest birds are absent. The few that were present were recorded in only one or a small number of the study localities, despite the fact that the same habitats were found in practically all of them. The absence of these species and the isolation of the few remaining ones is due to processes prior to the implementation of oil activity. In this sense, the construction of new infrastructure and the adaptation of the existing one will not imply a significant reduction of the remaining forest areas which, according to current regulations, must be protected. Conversely, if the forest offsetting process required for oil exploration and exploitation is well directed, this could result in an increase in the connectivity of the remaining forest fragments, favoring species mobility and a possible increase in diversity.
Keywords: birdlife, amazon, connectivity, conservation, oil exploration
Habitat degradation caused by human activities is a global problem. Erosion, water and soil contamination, deforestation, forest fragmentation, and loss of biodiversity are well-known consequences of processes such as expansion of the agricultural frontier, establishment of pastures for cattle ranching, and implementation of various industrial activities such as oil exploration and exploitation among others.1–3 In addition, these activities not only result in changes in the composition and structure of biological communities,2 but also in cultural and linguistic erosion among indigenous peoples,4 and in socio-economic conflicts between local indigenous communities and states.5–8
This is particularly relevant in megadiverse countries such as Colombia, which is home to the largest number of bird species worldwide and is among the most diverse in relation to biological groups as amphibians and fishes. In this sense, one of the regions with the greatest biological and cultural diversity in the world is the Amazon,9 and in Colombia, Putumayo and Caquetá departments in particular, where Andean and Amazonian species converge biogeographically. Many of these are endemic to Colombia and/or are at risk of extinction.10 Despite their great ecological and cultural importance, Caquetá and Putumayo have been subject to widespread processes of deforestation and ethnic erosion.11,12
In the case of western Amazonia, in addition to its huge biodiversity,13 there is an enormous wealth in the subsoil in the form of hydrocarbons, specifically oil,14 and minerals like gold and copper. In this region, oil exploitation has concentrated in the neighboring countries of Ecuador, mainly in the province of Napo where it has played a key role in the transformation of the landscape since the 1980´s,15 and Peru where about 70% of the Amazon has been assigned to hydrocarbon industry concessions.9 As it has happened worldwide, in these two Amazonian countries, oil exploitation has resulted in extensive deforestation, forest fragmentation, soil compaction, and water and soil contamination.1,3,15 However, this is not only the result of oil activity per se, but also and mainly of secondary effects associated with infrastructure construction and development,16 lack of state-of-the-art engineering, and not taking into account the social and environmental costs.17 Road construction is of particular importance because it gives access to previously undisturbed forests, accelerating colonization, deforestation and fragmentation as well as illegal poaching and illegal wildlife trade.2 However, although oil exploitation has had negative effects on Colombia´s Amazonian forests and biodiversity, it has not been the main determinant of its loss. Since the end of the last century, years before the exacerbation of oil exploration an extraction, forest loss has been concentrated in the so-called Amazonian arch of deforestation, towards the western portions of Putumayo, Caquetá, and Guaviare, reaching higher rates of deforestation than those in Ecuador and Perú.12 In this region the main causes of forest clearing have been the implementation of coca crops for illicit use and of pastures for livestock farming and, to a lesser extent, of banana and cereal monocultures and timber extraction.11,12 Thus, prior to the construction of the exploratory drilling area project in the Coatí block referred to in this paper, the area had already been extensively deforested and the landscape was already dominated by crops and pastures.18,19
Very little is known about the negative or positive impact that the oil industry may have on previously deforested areas,20 where reforestation and restauration projects can be strategic for restoring ecosystem structure and function.3 These could be done in key places that promote connectivity between the remaining forest patches that still remain in the landscape. In this paper we explore the possible effects that forest offsetting plans may have as a conservation and connectivity strategy in previously deforested and fragmented landscapes, in this case in the Coati exploitation block in the department of Putumayo in the Colombian Amazon.
Study area
Field work was carried out in the Coati block, an oil exploratory area of 23,064.51 ha located in the municipalities of San Miguel and Valle del Guamuez in Putumayo department (Figure 1), between the Andean foothills and the Amazonian plain,21 within Putumayo Pleistocene refuge.22 The area is dominated by hills and terraces, with a relief ranging from flat to slightly undulated terrain and moderate slopes of less than 30% and elevations between 250 and 350 m.a.s.l. The average annual temperature is 25°C, and average annual rainfall is of 3250 mm with two rainy seasons from March to May and from October to January.21 The rural area of San Miguel has been subject to intense anthropic activity including colonization processes, oil exploitation, implementation of extensive areas of illicit crops, and establishment of pastures for livestock, which have produced damage to soils, watersheds, forests, flora, and fauna.21 Coca crops for cocaine production account for the largest percentage of cultivated land, followed by cocoa, pineapple, plantain and cassava. The landscape in the area is composed of a mosaic of different vegetation covers, where crops and pastures predominate (Table 1).

Figure 1 General location of the Coati block and the Coati exploratory drilling area in the municipalities of San Miguel and Valle del Guamuez in the department of Putumayo in Colombia. Source: BIODER SAS.
|
Plant cover |
Influence area |
|||
|
Area (Ha) |
% |
Area (Ha) |
% |
|
|
Terra firme dense forest |
972,75 |
3,53 |
261,47 |
1,13 |
|
Fragmented forest |
2035,21 |
7,39 |
1750,42 |
7,59 |
|
Secondary vegetation |
10060,47 |
36,52 |
8557,99 |
37,11 |
|
Permanent crops |
11,64 |
0,04 |
11,64 |
0,06 |
|
Other crops |
12,63 |
0,05 |
12,63 |
0,05 |
|
Hydrocarbon extraction |
0,72 |
0 |
0,72 |
0 |
|
Wetlands: rivers, lakes, swamps, ponds |
144,02 |
0,51 |
117,93 |
0,51 |
|
Pastures with natural spaces |
430,26 |
1,56 |
414,04 |
1,8 |
|
Wooded pastures |
348,17 |
1,26 |
288,68 |
1,25 |
|
Palm stands |
0,13 |
0 |
0,13 |
0 |
|
Paddocks |
13444,04 |
48,8 |
11598,01 |
50,29 |
|
Urban area |
90,41 |
0,33 |
50,84 |
0,22 |
|
Total |
27550,47 |
100 |
23064,51 |
100 |
Table 1 Area and percentage of the total area of each type of vegetation cover found in the hydrocarbon exploration area in the Coati block and its area of influence using the Corine Land Cover methodology. Modification of the PMA Environmental Management Plan for the exploratory drilling of the Coatí 1 well (2013)
Valle del Guamuez is located north of San Miguel and encompasses areas inside and outside Coati´s oil exploratory polygon. Landscape within the oil exploratory area has also been subject to extensive deforestation but, outside, the largest patches of mature forest that remain in the region are found. These are located the indigenous reserves of Santa Rosa del Guamuez, Yarinal–San Marcelino and Campo Alegre–El Afilador. In 2013, a prior consultation process was carried out to obtain authorization to undertake an Environmental Impact Study and an avifauna inventory in the area.19
Coati´s avifauna
Species richness and composition
Bird species richness was estimated combining three complementary methods:
1) Observation and auditive transects
2) Mist net captures
3) Ornithological ethno-inventories
Transects and captures were made in eight locations around Coati exploratory drilling area (Figure 2), encompassing all habitats and vegetation types found in each one. Transects were performed in the mornings between 5:00 a.m. and 11:30 a.m., and in the afternoon between 2:00 p.m. and 5:00 p.m. Captures were made only during Coati´s Conservation Plan for Threatened Birds development. At each sampling site 12 x 2.5 m mist nets were installed, in a consecutive or interrupted arrangement depending on the level of bird activity. Mist nets were opened between 6:00 a.m. and 11:00 a.m. and between 1:00 p.m. and 3:00 p.m. Captured birds were identified, photographed, and released. In each case notes on the type of vegetation in which birds were recorded were taken.
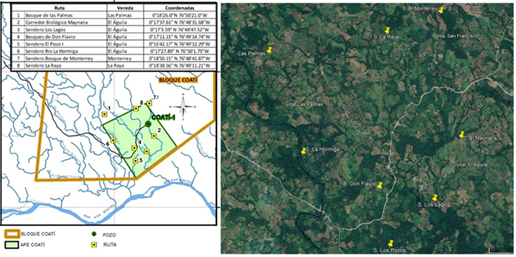
Figure 2 Location of the eight observation sites within the area of the Coati exploratory block. (Source: BIODER SAS) (A), and the vegetation cover in each of them (Google Earth satellite image) (B).
Ornithological ethno-inventories were performed among older local inhabitants and Coati´s workers by showing pictures of bird species potentially distributed in the area. When a species was identified as being present in the area, we registered its common name, habitat, seasonality and economic and cultural importance. This allowed us to identify seasonal or migratory species, which are not present throughout the year and could not possibly be observed during field work, as well as some birds as parrots and eagles among others, that are well-known and may have been overlooked due to the limited time scope of the study.
Observation and auditive transects, captures, and ethno-ornithological inventories were done during three moments that took place between November 2012 and May 2019:
-The formulation of the Environmental Management Plan for the exploratory drilling of the Coati well, between November 23 and 27, 2012.18
- The formulation of the Environmental Impact Study for the Coati hydrocarbon exploitation area, between November 29 and December 10, 2013.19 During this visit it was impossible to carry bird inventory according to the established methods because of security restrictions and therefore it was impossible to make observations after 3 p.m. There was no access to the most preserved forest patches because there were coca crops for illicit use there, and it was not possible to enter the villages of Campo Bello, Pavas Altas, Pavas Bajas and Llano Verde.
- Coati´s Threatened Bird Species Conservation Plan development, between April 17 and 21 and between May 10 and 11, 2019.23
Threatened and/or commercially valuable species
Threatened species were established according to the latest edition of the Red Book of Birds of Colombia,10 the most up-to-date Bird Guides of Colombia24 and the IUCN red lists of threatened species.25,26 It´s important to notice that between the first 2019 review and the second one in 2023, the status of several species changed due to increases in their populations and in our knowledge of their distribution and abundance. On the other hand, we used CITES appendixes,27 to determine threatened or nearly threatened species due to illegal trade. These include species whose trade is completely prohibited (Appendix I), species whose trade is subject to strict regulations (Appendix II), and species that are included at the request of a party that already regulates their trade and that need international cooperation to prevent their unsustainable trade (Appendix III).
Migratory species
Boreal and austral migrants were determined according to Colombia´s Guide to Migratory Species,28 and the most recently published Bird Guides of Colombia.24
Avifauna
Overall, in the eight localities associated with Coati´s exploratory drilling area, we recorded 180 bird species distributed in 138 genera, 44 families, and 21 orders. Avifauna composition is characteristic of forests and other Amazonian lowland ecosystems where Tyrannidae (flycatchers), Thraupidae (tanagers and allies), Thamnophillidae (antbirds), and Furnariidae (ovenbirds and woodcreepers) represent the largest number of species. The low number of species of families such as Accipitridae (eagles) and Psittacidae (parrots, parakeets and macaws), generally very diverse in Amazonian ecosystems, was notorious. The same was observed about the high species richness of Icteridae (oropendolas and caciques) and Columbidae (pigeons), among others, which are more diverse in disturbed environments.
Bird diversity by habitat and sampling Location
The bird diversity found in the study is the result of the sum of the species recorded in each of the six habitats sampled (Annex 1). Within these, forest ecosystems, wetlands (streams, rivers, lakes), and open areas contributed the greatest richness to the total diversity (Figure 3A & 3B). Twenty-three of the recorded species were exclusive to forests, while seven of the open area species were not observed in any other habitat. In wetlands, the total number of species was not high (37), but a high proportion of them was exclusive to aquatic environments and was not recorded in land-based habitats. In any given locality, we recorded between 25 and 78 bird species. Maynana sector had the highest bird diversity, and the La Raya Trail had the lowest (Figure 3B). According to the similarity among the birds recorded, the localities can be classified into four groups: 1) Las Palmas, Maynana Corridor, Monterrey Forest and La Hormiga Trail, had the most similar avifauna, followed by 2) Don Flavio Forest and Los Pozos, 3) the Lakes, and 4) the La Raya Trail (Table 2). In the four localities of the first group there is a greater amount of forest and, consequently, of bird species associated with wooded environments; in the second group, the two localities are geographically very close to each other; the third group is where there is the greatest area of aquatic ecosystems, and the fourth corresponds to the locality with the lowest number of species.

Figure 3A Total number of species and unique species recorded in each of the habitats in which observations were made.

Figure 3B Number of species recorded in the habitats present in each of the eight sampling localities.
OA; Open areas, St; Stubble, FB; Forest Border, Fo; Forest, RF; Riparian Forest, W; Wetlands, ES; Exclusive species.
|
Palmas |
C Maynana |
Los Lagos |
B Flavio |
El Pozo |
S. Hormiga |
B.Monterrey |
S.Raya |
|
|
Palmas |
75 |
0,55 |
0,41 |
0,48 |
0,48 |
0,50 |
0,53 |
0,34 |
|
C Maynana |
42 |
78 |
0,36 |
0,44 |
0,46 |
0,28 |
0,47 |
0,31 |
|
Los Lagos |
23 |
21 |
38 |
0,38 |
0,29 |
0,47 |
0,38 |
0,25 |
|
B Flavio |
34 |
32 |
20 |
66 |
0,37 |
0,42 |
0,52 |
0,35 |
|
El Pozo |
32 |
31 |
14 |
23 |
58 |
0,40 |
0,36 |
0,31 |
|
S. Hormiga |
37 |
41 |
26 |
29 |
26 |
73 |
0,41 |
0,41 |
|
B.Monterrey |
35 |
32 |
18 |
32 |
21 |
27 |
58 |
0,41 |
|
S.Raya |
17 |
16 |
8 |
16 |
13 |
17 |
17 |
25 |
Table 2 Sorensen similarity index between pairs of localities in which avifauna inventories were made. The diagonal shows the number of species recorded in each locality. Above the diagonal is the value of the index, and below is the number of species shared between pairs of localities
Important species
- Conservation
In Coati´s 2019 Conservation Plan for Threatened Bird Species.23 three species were listed as vulnerable to extinction at global level:25 the whistling toucan Ramphastos culminatus (Linnaeus, 1758), the white-breasted toucan Ramphastus vitellinus (Lichtenstein, 1823), and the red pigeon Patagioenas subvinacea (Lawrence, 1868). However, since then, the populations of these species have increased, and none of them are currently at risk of extinction at a global or national level.26 Likewise, none of the birds recorded is endemic to Colombia.
- Wildlife trade
None of the birds recorded is at risk due to wildlife trade.27 However, all hummingbirds, eagles, owls, hawks, parrots, macaws, and three species of toucan could become threatened if trade is allowed with no control (Appendices II and III).27
- Migratory birds
Eight species are seasonal migrants to the study area. Six are boreal migrants: Tringa solitaria (Wilson, 1813) (Scolopacidae), Elanoides forficatus (Linnaeus, 1758) (Accipitridae), Empidonax alnorum (Brewster, 1895) and Contopus virens (Linnaeus, 1766) (Tyrannidae), Vireo flavoviridis (Cassin, 1851) (Vireonidae) and Setophaga fusca (Müller, 1776) (Parulidae), and two are austral migrants: Empidonomus varius (Vieillot, 1818) and E. aurantioatrocristatus (d'Orbigny & Lafresnaye, 1837) (Tyrannidae).
Coatí´s avifauna is characteristic of Amazonian lowland forests where a few families, especially of insectivorous and frugivorous birds, have the highest species richness and represent the greatest diversity.13,29 However, the composition of these families is different from the composition in well-preserved Amazonian forests. This is similar to what has been found in other Amazonian areas where landscape transformation has had no major effects on functional diversity but has had great impact on taxonomic diversity and bird species composition.30 In first instance, the number of species of each of these families is much smaller, and all of them lack some common species and even genus associated with well-preserved forests.29,31 Within these, there are representatives of guilds such as understory insectivorous or game birds, which are highly affected by deforestation, fragmentation and poaching.32–34 Among the flycatchers, the complete absence of species of Lophotriccus, Inezia, Sublegatus, and Zimmerius and others, was noticeable. Among the antbirds, Gimnopithys, Willisornis, Myrmeciza, or Hylophylax, many of which are abundant in other localities, were not reported. On the other hand, the absence of ovenbirds and woodcreepers genus like Xiphocolaptes, Xiphorhynchus and Xenops indicates that the species richness of this family in the study area is much lower than in the preserved forests of other Amazonian areas. In addition, the low number of eagles, parrots, parakeets and macaws, as well as of other groups associated with mature forests such as curassows, also reflects that there are only a few small isolated relicts of forest immersed in a matrix of pastures and crops that are not suitable habitats for most forest specialists.34,35–37 This is also evidenced by the relatively high species richness of families such as Icteridae (caciques) and Columbidae (pigeons); many of them are associated with anthropic environments.
In this sense, it is evident that the forests in the area present a high level of alteration in their composition and structure,21 which leads to a disconnection and alteration of biodiversity reflected in the low richness of forest bird communities. In fact, there are no primary forests in the area. However, in practically all eight sampling locations, there are relicts of secondary forests and young stubble. Although these are highly disconnected and isolated, it is undeniable that together they make important contributions to overall bird diversity in the area.36,37 If these could be connected to each other and then to large forests, as those in the indigenous reserves, this would allow the mobility and probably the immigration of species, thus increasing richness and diversity in the landscape.
Despite the absence of most forest and other mature ecosystems bird specialists, some of the species recorded are bioindicators, important components of local communities’ diets, and target taxa for recreational birdwatching.30 This is the case of tinamous and guans, hunted for meat by many Amazonian communities, which has led to the decline of their populations in several localities. On the other hand, most antbirds were exclusive to the few forests fragments that remain in the area. Antbirds are indicators of relatively well-preserved habitats and are strongly affected by deforestation and forest fragmentation.32,38 In fact, they are among the first to disappear after disturbance, and they are reluctant to cross small clearings even of the width of a road. On the other hand, in these habitats we found species such as the Rufous-headed Woodpecker (Celeus spectabilis (Sclater and Salvin, 1880)), which in Colombia has only been recorded in this region and is highly valued by birdwatchers.
At each locality, species richness was associated with the relative amount of forest in relation to other habitats, which in turn was reflected in the number of unique species in each habitat and in the similarity between the birds recorded there. However, despite their geographical proximity and the fact that practically all localities have the same habitats, the similarity between them was quite low (Table 2). With the exception of the La Raya Trail, between 4 and 14 species were exclusive to each locality, for a total of 71 species that were reported only in one of the eight localities (40% of all the species reported for the Coatí block). This indicates that the inventory of birds present in each locality is far from complete and that, if the sampling effort was increased, the avifauna of the localities would be much more similar to each other.
For example, three of the four recorded eagles: Leptodon cayenensis (Latham, 1790), Elanoides forficatus and Ictinia plumbea (Gmelin, 1788), were only reported on the La Hormiga Trail, despite the fact that they are generalists, have great mobility, and could easily move between localities.24 The same happens with the two species of toucan of the genus Ramphastos: R. tucanus and R. vitellinus, which were only recorded in three of the eight localities despite the fact that, in other Amazonian areas, they have been seen making large movements between forests in different state of conservation.29,39 Similarly, Celeus spectabilis, Micrastur buckleyi (Swann, 1919), and M. semitorquatus (Vieillot, 1817), a woodpecker and two medium-sized falcons associated with riparian forests, are known to make long distance journeys. Despite being mainly associated with the forest interior, they could fly to locations different from those where they were recorded. On the other hand, species such as Sclateria naevia (Gmelin, 1788) and Taraba major (Vieillot, 1816), two antbirds specialized in riparian and terra firme forest undergrowth respectively, are strongly affected by fragmentation processes and find it difficult to cross small clearings.32
None of the recorded species is endemic to Colombia, is at risk of extinction,10,26 or is threatened by illegal wildlife trade.27 However, the presence of endemic and/or endangered species in other nearby Andean-Amazonian areas,30,31 and in lowland Amazonian ecosystems29 suggest that these were probably present in the area before the intensive and extensive deforestation that occurred mainly in the last century.12 In this way, an eventual negative impact on the avifauna caused by oil exploration and extraction would not include highly important taxa in terms of conservation (endangered or endemic species), which had already been lost due to the expansion of the agricultural frontier and the establishment of illicit crops.11,12 On the other hand, none of the recorded bird species are threatened due to illegal wildlife trade, but there are groups of birds that could become so such as parrots, macaws, eagles and toucans among others.27 In this sense, several studies have shown that illegal pouching has increased with the opening of new roads for oil exploration and exploitation. However, this has occurred in places with large areas of pristine forest where roads did not previously exist, and their opening gave access to previously inaccessible forested areas.2,16 On the contrary, in the study area, no primary forests remained when the oil exploration project began, and practically all access routes already existed.18,19
However, some of the species recorded, despite not being on the red lists, can be important in terms of conservation and food security for local communities. This is the case of the guan Pipile cumanensis (Jacquin, 1784), recorded in Don Flavio Forests and the three tinamou species recorded in the Maynana biological corridor: Crypturellus soui (Hermann, 1783), C. cinereus (Gmelin, 1789) and C. undulatus (Temminck, 1815), which are widely consumed by local communities in the entire Amazon.40 Most of these species were recorded only in one of the eight locations, with small populations that may be undergoing hunting processes, so conservation actions should be implemented. Migratory birds were also poorly represented in the study area. This is due to the fact that field work was carried out towards the last and the first months of consecutive years, during the boreal migration when migrants leave their breeding grounds in North America and head towards their migratory areas in tropical ecosystems.28 Practically no observations were made in the middle of the year, when the southern migratory species arrive in the country. It is also significant that, in the area, there are no large rivers in which beaches form during the dry season. Beaches are preferred habitats for several plovers (Scolopacidae) and other migratory birds. Additionally, the sampling effort was not sufficient to detect low abundance and/or inconspicuous species as several warblers (Parulidae) and vireos (Vireonidae).
It is highly probable that the diversity of migratory birds in the area is much greater than the one found in this study. There are several unrecorded migratory species which must be present in the study area. Some of these are common in intervened habitats24 as those found in the eight localities, while others require forest cover that provide shelter and food along their migration routes.39 Forest patches in the area could fulfill this function. It is possible that migratory birds not registered include endangered species. If their presence is confirmed, this would provide further evidence of the importance of at least preserving the few remaining forest fragments. The low diversity of birds recorded in the study area reflects the extensive fragmentation to which the forest has been subjected and the high isolation of the small remaining forest patches. However, this does not mean that management plans should not be implemented for the conservation of some important species, as well as birdlife in general. On the contrary, it is imperative to carry out actions aimed at the protection of the fauna that still subsist, and mainly to improve the ecosystem services on which it depends. In this sense, the offsetting required for oil exploration and drilling processes can be strategic and should be focused on restoration processes. First of all, selected plant species should have ecological and physiological characteristics that allow them to resist and survive adverse conditions as those found in pastures for cattle ranching.3 Second, there must be clear objectives in terms of connectivity understanding that the effects of fragmentation not only depend on factors such as the size, shape, and isolation of forest patches, but also in the vegetation matrix that surrounds them and on the way in which secondary growth recovers over time.41,42
In this sense, it is necessary to ensure that the remaining patches, and secondary forests, have the conditions to support not only diversity but also population processes and emerging community properties. First of all, increasing the size of the largest fragments should be looked for, so that they reach a minimum area required to avoid large biodiversity losses.34,41 This can be accomplished focusing restoration on forest borders. Likewise, many species avoid clearings of more than 100 m wide, so it is strategic to ensure connectivity between major forest patches, and in general increase all fragments connectivity. For this purpose, restoration should be directed towards river banks and other landscape elements that can act as biodiversity corridors. Larger and more connected fragments would allow species movements and gene flow. Thus, even isolated species as those found in the study area could leave the areas in which they are confined and colonize other forest patches.
On the other hand, it is necessary to understand that restoration processes not only depend on biological and ecological factors, but also largely on social and economic variables. Farmers, ranchers, and other stakeholders depend economically on the productivity of their land, so successful restoration must take into account an alternative economic development of the region. There is no point in asking them to change their economic activities or even to reduce the crops or ranching areas if there are no livelihood alternatives. Plants used in restoration, mainly for the matrix that surrounds forest fragments, must include fruit, timber, and forage species that may have a direct and positive impact on persons and livestock. We recommend to negotiate with farm owners to implement agroforestry and silvopastoral systems as part of the offsetting required for hydrocarbon projects. These are well known to have positive effects on biodiversity,37 including forest species that tend to disappear faster and that may be at risk of extinction,43 as well as on farm economic productivity.
Finally, it is advisable to use "best practices" to reduce the impact generated by hydrocarbon exploration and exploitation projects. These should include the use of state-of-the-art engineering and take into account environmental and social aspects and costs,17 which are generally not taken into account because they are supposed to raise the costs of production, which is not necessarily true.
The composition of the avifauna in the Coati area of exploratory drilling interest was characteristic of Amazonian secondary lowland forests, with very low presence of forest specialists and a complete absence of endemic or endangered species. This was to be expected according to the intensive deforestation to which the region has been subjected due to the implementation of monocultures, pastures for cattle ranching and crops for illicit use. This occurred before the implementation of oil activity, so that the impacts that it may have on the diversity of birds present in the area are very low and will not include important taxa in terms of conservation.
It is known that patches of secondary forest have the capacity to sustain a bird diversity similar to those of well-preserved forests. However, in the study area the high degree of fragmentation has exceeded the level below which significant biodiversity losses occur, and the few characteristic remaining forest specialists are isolated in small patches that have completely lost their connectivity. Compensation, restoration and reforestation processes required for oil exploration and exploitation projects must be directed to increase the area of the remaining forest fragments and generate connectivity between them. These should also include the implementation of agroforestry and silvopastoral systems that enhance the productivity of productive systems, while increasing wooded vegetation in the pasture matrix that currently dominates the landscape. In this way, species movement and gene flow would occur not only between forest patches, but also each time further into degraded or transformed habitats, facilitating the succession of bird communities and the recolonization of forested areas, thus reversing the process of fragmentation and the consequent loss of biodiversity. In this sense, it is important that environmental impact studies such as the one carried out by oil companies in heavily degraded areas before the entry of exploration/exploitation, not only evaluate the negative impact that their activity will have on the wildlife that subsists in the area, but also the positive impacts that the implementation of well-designed compensation plans can have on biodiversity.
If some of the above recommendations are taken into account, hydrocarbon exploration or exploitation projects must not necessarily result in biodiversity and forest loss, in particular in landscapes where most deforestation has occurred prior to their arrival. In fact, in these areas, well directed compensation, restoration and reforestation processes should result in healthier and more connected forest fragments in which bird diversity could recover. The oil industry not only has the economic and political capability to do so, but also a huge social and ecological responsibility.
Biologists Alexis Calderón, Gerardo Delgado Bermeo and Camilo Andrés Yasnó, contractors of Bioder SAS, carried out the inventory of the birdlife within the framework of the Conservation Plan for Threatened Species in the Coatí Block. Luisa María Delgado made a first translation of the Spanish manuscript.
The authors declared that there are no conflicts of interest.

©2024 Carrillo-Chica, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.