International Journal of
eISSN: 2574-9862


Research Article Volume 3 Issue 3
1Faculty of Veterinary Science, Universidad Nacional de La Plata, Argentina
2National Scientific and Technical Research Council, Argentina
Correspondence: Nora Mestorino, Laboratory of Pharmacological and Toxicological Studies (LEFyT) Veterinary Faculty, Universidad Nacional de La Plata, Street 60 and 118, w/n 1900 La Plata, Argentina
Received: May 25, 2018 | Published: June 29, 2018
Citation: Mestorino N, Zeinsteger P, Buchamer A, et al. Tissue depletion of doxycycline after its oral administration in food producing chicken for fattening. Int J Avian & Wildlife Biol. 2018;3(3):245-250. DOI: 10.15406/ijawb.2018.03.00095
Doxycycline (DOX), tetracycline of second generation, is mainly active against Gram–positive and Gram–negative bacteria, aerobic and anaerobic. Although there are few pharmacokinetic studies in chickens, it is frequently used for the colibacillosis treatment, salmonellosis, staphylococcal infections, avian mycoplasmosis and chlamydia. The objective of this study was to evaluate the withdrawal time (WT) of DOX formulation at 25 % in edible tissues, after its oral (PO) use in broilers. Forty healthy chicks (30–35 days of age) were used. DOX was administered with drinking water for 5 days at 10 mg kg–1 (N = 36); four untreated animals were reserved (control). Six animals per group were euthanized by cervical dislocation after desensitization by passage of an electric current through the head, after 24 hours until 9 d post treatment and control animals also. Muscle, liver, kidney and skin/fat samples were obtained. DOX was determined by HPLC with UV detection. DOX concentrations were determined in all tissues examined; generally falling below the MRL at 7 d after administration is terminated. It was estimated 6.58, 8.18, 8.69 and 6.96 d of WT for muscle, liver, kidney and skin/fat, respectively. After DOX administration at a rate of 10 mg kg–1 for 5 days in drinking water, a WT of 9 d is suggested in poultry destined for human consumption.
Keywords: antimicrobials, doxycycline, poultry, tissues, residues, withdrawal time
Poultry production in Argentina has grown during last years because of an increase in consumption of poultry and poultry products. Chicken consumption increased from 20 kg/person/year in 2000 to 44 kg for the last years.1 Due to the increase in demand of animal protein in population’s diet, important investments were implemented in the poultry sector by means of technological improvements, genetics, animal welfare, health and nutrition, which in turn contributed to the development and intensification of this activity. For this reason, activities related to poultry production are betting to include new technologies, extreme quality and sanitary controls. An example of this is the control of residues and contaminants that exceed those values allowed in the meat made by national inspection bodies in order to take care on behalf of consumer health, to punish the noncompliance of current regulations and to maintain a free market to export products produced in Argentina.
Tetracyclines are the most commonly used antimicrobials in food–producing animals. They are broad spectrum antimicrobials widely used in poultry farms because of their great activity against Gram–positive and Gram–negative bacteria, and intracellular microorganisms. Doxycycline (DOX) is a semi–synthetic bacteriostatic tetracycline which tends to be more active against the spectrum of microorganisms, including Rickettsiae, Chlamydiae, Mycoplasmas and some Protozoa.2,3 Therefore, DOX is used for the treatment of respiratory, urinary and gastrointestinal tract diseases.4
Pharmacokinetics properties of DOX are superior than older tetracycline; in terms of higher lipid solubility, complete absorption, better tissue distribution, longer elimination half–life and lower affinity for calcium.5,6 It has good bioavailability after oral administration, and it is widely distributed in the body. Plasma protein binding is greater than other tetracyclines and all these characteristics contribute to maintain plasma concentration for long periods requiring less frequent doses than other members of the tetracycline family.7,8 The route of drug administration plays an important role in the effectiveness of the treatment, as well as in the distribution of antibiotics to tissues.9
The prudent use of antimicrobials in poultry industry results in many benefits. Antibiotics are used in chickens to enhance growth, feed efficiency and reduce diseases. Additionally, prophylactic treatment is common during periods of stress. Although there are few pharmacokinetic studies in chickens, it is frequently used for the treatment of colibacillosis, salmonellosis, staphylococcal infections, avian mycoplasmosis and chlamydiasis.10
Some antimicrobials may leave residues in several tissues and animal products. In Argentina DOX is frequently used in poultry production; therefore, it is important to control its residues in edible tissues. Doxycycline given to chickens orally raise possibility for residues which may remain in edible tissues, particularly when animals are slaughtered without respecting the withdrawal period. There are several factors which may increase the risk of accumulation of DOX in poultry tissues such as imprudent use for overdosing, misuse, insufficient withdrawal time and failure in the doses. Such residues may cause public health hazards to consumers including toxicological, microbiological, immunological and pharmacological disorders depending on the type of food and the amount of residue present.11
Residual levels of DOX in animal tissues have several potential risks for consumers. One of them is the potential hazard to select resistance in commensal strains of medicated animals. Those selected resistant commensal strains become a problem because the plasmids containing resistance genes could be transferred to human bacteria.12 Additionally, bacterial populations do not respond to traditional treatments commonly used for human illnesses.13
The antibiotics’ residue levels reached in organs and the rate of their depletion from tissues depend on the method of administration, animal species, as well as dose and specific formulation of a given drug. The differences in the antibiotic concentration and time of its depletion may also be influenced by the differences in the intake of drinking water by animals as well as its quality. Studies on tissue concentrations after different drug formulation administration are essential to control antibiotic residues in food animal products, in order to recommend the appropriate withdrawal time.
The European Commission has established limits for maximum residues (MRLs) allowed in foodborne products from poultry industry, in muscle the MRL is 100 μg kg–1; 300 μg kg–1 in liver and skin/fat and 600 μg kg–1 in kidney. According to the Commission Regulation14 and EMA Summary Report Doxycycline 2, quantification of DOX in animal tissues requires a determination of only the parent compound as the residue marker, without its 4–epimer, contrary to other tetracyclines for which the MRLs are defined as a sum of parent drugs and their 4–epimers (EU N° 37/2010, EMEA/MRL/270/97, 1997). The purpose of the present study was to evaluate the withdrawal time (WT) of experimental DOX formulation at 25% in edible tissues, after its PO use in broilers.
Study location
Experimental animals were provided by a farm located in Buenos Aires province, Argentina. All the in vivo assays were performed at the Laboratory of Pharmacological and Toxicological Studies (LEFyT,)–School of Veterinary Science, National University of La Plata, Argentina.
Experimental formulation
Doxycycline was formulated as a 25% experimental water soluble powder (DOX 25g, Tartaric acid 5g and Lactose sqt 100g).
Sixty healthy BB chickens destined for fattening were kept for 30 days with water and food free of any type of antibiotic.
The determination of individual daily water consumption is complicated in chickens and in general results in unreliable data. The control of water consumption, therefore, was made with the experimental group constituted in its entirety. The animals were fed with balanced feed and water "ad libitum". To know the real amount of water consumed daily, a trial was performed five days before the initiation of the treatment with cubed drinkers graduated. Consequently, water ingested was measured day by day for five consecutive days. In this way it could be ensured that the water ingested daily was that which would correspond to the dose of the medication that would be administered in the assay in order to decrease the risk of under dosing.
The experiment was conducted on 40 five–week–old chickens, 36 animals were treated with 10mg kg–1 body weight of DOX (25%) once a day, during five consecutive days with drinking water. Solution was prepared by dilution of 400 g of the medicament in 1000mL water. The solution was prepared in a base of a daily use and containing the necessary amount of antimicrobial for an exact dosage. After that, the next day, the water administration system was completely emptied, washed and a new solution was prepared. The same procedure was repeated until day five. After the finalization of the antimicrobial administration, the system was suitably washed and loaded with water free of any chemical compound.
The birds were kept in a special space designed for performing experiments on animals and before treatment they were deprived of water. Antibiotic free food was available ad libitum.
Chickens treated with DOX were euthanized by cervical dislocation after desensitization by passage of an electric current through the head 1d, 2d, 3d, 5d, 7d and 9d after the last administration (six animals at each time point) and then muscle (breast), liver, skin plus fat and kidney were collected. Four chickens used as controls were euthanized before the experiment, and same tissues samples were collected. All samples were kept separately at–20 °C until analysis.
The protocol was according to the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (Federation of Animal Science societies –FASS–) and was approved by the Experimental Ethics Committee of the Faculty of Veterinary Science, UNLP, Argentina.
Reagents
Doxycycline (DOX) standard was obtained from Sigma–Aldrich Chemical Company (USA). Acetonitrile and methanol were from J.T. Baker. Oxalic acid dehydrate, trichloroacetic acid and sodium sulphate anhydrous were from Fluka (USA). Solid phase extraction (SPE) columns (Strata C18, 100mg, 1mL) and analytical column (Luna C18) were obtained from Phenomenex (USA).
Standard solutions
Stock standard solution (1mg mL–1) was prepared by weighing 10.0±0.1mg of standard substances and dissolving in 10mL of methanol. The stock solution was stored at –20 ºC in amber glass and was stable for six months. Secondary standard solutions (100μg mL–1 and 10μg mL–1) prepared in methanol by diluting suitable aliquot of stock standard were stable for one month, stored at 2–8 ºC in amber glass. Working standard solutions in mobile phase were prepared on the day of analysis.
Extraction and clean–up
A sample of 0.4 g tissue (problem or spiked with DOX) was homogenized with 1.4mL of Mc–Ilvaine buffer–EDTA (pH 4), shaken at high speed, and centrifuged at 2.500 g at 4 °C for 15 min. The upper layer (supernatant S1) was transferred into a new tube. The extraction was repeated three times. The supernatants S2, S3 and S4 were coupled with S1. The mixture was vortexed for 30 s and centrifuged again for 10 min at 2500g at 4 °C. Mc–Ilvaine buffer–EDTA (pH 4) was prepared by dissolving 15 g of disodium monohydrogen–orthophosphate dehydrate, 13g of citric acid and 3.72g of EDTA in water and diluting to 1L.
Cleanup
The supernatant mix (S1–S2–S3–S4) was transferred to SPE C18 cartridges, which were preconditioned with 3mL of methanol and 2mL of ultrapure water. The tube reservoir mix supernatants were washed with 1mL of McIlvaine buffer–EDTA and 1mL of water, after percolation of the whole solution; the columns were washed with these solutions (under vacuum). After drying for 2 min, the doxycycline was eluted with 4mL of methanol 0.01 M oxalic acid pH 2.0. The cleaned extracts were evaporated to dryness in nitrogen evaporator at 40 °C. The dried residues were reconstituted in 200 µL mobile phase. After vortexing and centrifugation, 100µL were injected into the chromatographic system.
LC–UV analysis
The instrumental analysis was performed using Gilson HPLC system, equipped with isocratic pump, autosampler, column oven, and UV/Vis detector (λ=346 nm), controlled by Unipoint Workstation software. Chromatographic analyses were performed on Luna (Phenomenex) C18 column (5μm, 150mm x 4.6mm) with mobile phase consisting of water–acetonitrile with 0.02 M oxalic acid and 0.0005M EDTA (72:28, v/v) at 1.2mL min–1 flow rate. The column oven temperature was controlled at 30 ºC.
Method validation
The following parameters were evaluated for the analysis of each matrix: linearity (concentrations of DOX ranging between 0. 1 and 6 µg ml–1 – µg g–1), precision and accuracy, limit of quantization, limit of detection and selectivity. The standard calibration curve was prepared by the injection of standard solutions on seven levels (0.1, 0.2, 0.5, 1, 2, 4 and 6 µg mL–1 or µg g–1). The correlation coefficient was evaluated.
Tissue samples were spiked with the DOX working solution to levels corresponding to 0.2, 0.5, 1 and 2µg g–1. Six spiked samples (of each tissue) with DOX were analyzed within three different days. Based on these spiked samples replicates, the precision (repeatability and reproducibility) of the method was determined. The mean accuracy (expressed as % recovery) was evaluated by comparing the concentrations in the spiked samples with known amounts of analyses to the concentrations in standard solution which should be within the range 85–115%, while the variation in precision should be≤20% (coefficient of variation–CV–). The limit of detection (LOD) was estimated through the analysis of 20 aliquots of control tissue (free of DOX). The noise of the base–line was measured; the average and the standard deviation were calculated. The LOD corresponds to three of those SD (sign/noise≥3/1) and the limit of quantization (LOQ) corresponds to ten of these SD (sign/noise≥10/1).
Withdrawal time
Numerous experimental designs and statistical approach are used to establish the withdrawal time. The EMA recommends the use of a linear regression analysis of the logarithmic transformed data as the choice method.15 The withdrawal time is determined as the time when the one–sided, 95% upper tolerance limit of the regression line with a 95% confidence level is below the MRL. Doxycycline concentrations in function of time found in muscle, kidney, liver and skin/fat were plotted and analyzed with the program WT version 1.4 in order to recommend a period of withdrawal time for this experimental formulation.
The development and validation were successfully accomplished. This method performed accurately and reproducibly over a range of 0.1 to 6 µg mL–1 or µg g–1 for DOX.
Precision of the system
One standard solution was prepared containing 0.2µg mL–1 of DOX and the precision of the system was evaluated after the placement of twenty (20) injections in the chromatographic system. In this manner the efficiency of the column and of the system were evaluated. After twenty injections a coefficient of variation (CV) of 5.17% was determined.
Assay linearity
This assay exhibited a linear dynamic range between 0.1 and 6µg mL–1. A linear relationship was obtained across one dynamic range (r values ranged from 0.9923 to 0.9997) (Figure 1).
Specificity
Six different samples from control tissue (free of DOX) and 6 tissue samples fortified with DOX were analyzed by HPLC and the corresponding chromatograms were compared. No matrix interferences were observed on the chromatograms of the samples with the same retention time as doxycycline. The chromatographic analysis time was short and DOX was presented in 4 min as a sharp and symmetrical peak with no interfering peaks (Figure 2).

Figure 2 Chromatograms of chicken blank muscle and spiked muscle samples with DOX at levels of 0.2; 0.5; 1 and 2µg g-1.
Limit of detection (LOD)
The LODs were 0.040, 0.024, 0.024 and 0.010 µg.g–1 for DOX in kidney, skin/fat, muscle and liver respectively.
Limit of quantitation (LOQ)
The LOQs were 0.095, 0.074, 0.054 and 0.100 µg.g–1 for kidney, skin/fat, muscle and liver respectively.
Intra–day and inter–day recovery and precision
The method for the analysis of tissue samples was thoroughly validated and the results are presented in Table 1. To assess the inter–day (over 3 days) assay recovery and precision, 6 sets of tissue samples were prepared containing DOX at 0.2, 0.5, 1 and 2µg.g–1. The inter–day variation in recovery and precision were assessed. The mean recovery should be within the range 85–115 % and the variation in precision should be 20%. To determine the intra–day recovery and precision, 6 replicates of each 4 concentrations were analyzed along with duplicate standard calibration curves prepared from 2 separate stock solutions (Table 1).
Intra–day |
Inter–day (over 3 days) |
|||||
matrix |
r |
µg/g |
Recovery (%), n=6 |
Precision (%), n=6 |
Recovery (%) |
Precision (%) |
Muscle |
0.9949 |
0. 2 |
99.88 |
5.35 |
99.96 |
5 |
0.5 |
89.71 |
5.5 |
91.24 |
4.62 |
||
1 |
109.65 |
4.53 |
107.88 |
2.34 |
||
2 |
97.76 |
3.31 |
96.75 |
2.49 |
||
Liver |
0.9923 |
0.2 |
96.38 |
8.6 |
97.8 |
4.96 |
0.5 |
93.13 |
3.03 |
101.59 |
6.61 |
||
1 |
82.28 |
0.62 |
90.74 |
8.34 |
||
2 |
86.45 |
7.42 |
93.96 |
7.44 |
||
Kidney |
0.9931 |
0.2 |
97.5 |
3.54 |
104.05 |
13.03 |
0.5 |
93.96 |
5.66 |
94.37 |
2.72 |
||
1 |
106.5 |
2.12 |
98.47 |
8.39 |
||
2 |
97.75 |
1.06 |
98.85 |
1.04 |
||
Skin/Fat |
0.9951 |
0.2 |
89.09 |
0 |
86.36 |
2.73 |
0.5 |
98.91 |
0.03 |
98.97 |
3.03 |
||
1 |
105.46 |
0.07 |
99.49 |
5.78 |
||
2 |
98.36 |
0.1 |
96.12 |
2.55 |
||
Table 1 DOX recovery and precision intra–day and inter–day (3) from chickens simple tissues spiked with doxycycline
Doxycycline tissue concentrations
Doxycycline concentrations were determined in all tissues examined; generally falling below the MRL at 7 d after administration is terminated. The mean DOX tissue concentrations (±SD) obtained after the PO administration of doxycycline to chickens in muscle, liver, kidney and skin/fat tissues are shown on Figure 3. After DOX administration to chickens at the dose of 10mg kg–1 for five consecutive days through the drinking water, the highest content was found for kidney and liver at one day after treatment was completed. The maximum determined concentration in the kidney was 6.5μg g–1 and in the liver was 4.79μg g–1. On the other hand, muscle and skin/fat values were lower, with maximum concentration of 1.46μg g–1 and 1.23μg g–1, respectively. Subsequently a rapid decrease in DOX concentration was observed in all tissues. At 7 d after treatment was completed, the DOX concentration in muscle was above LOQ of the method used in this trial. Linear regression analysis of the logarithmic transformed data can be considered for the calculation of the withdrawal periods. Using this approach, the withdrawal time is determined as the time when the one–sided, 95% upper tolerance limit of the regression line with a 95% confidence level is below the MRL.
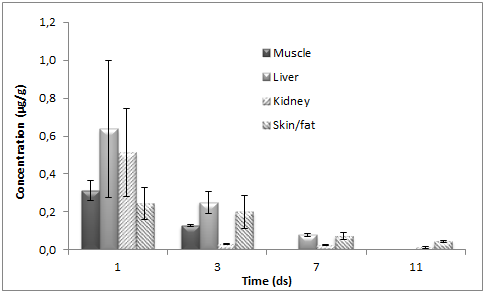
Figure 3 Mean (±SD) tissue concentrations of doxycycline in chickens slaughtered 1, 2, 3, 5, 7 and 9 d after oral administration of DOX 25% (dose of 10mg kg-1 body weight during 5 days).
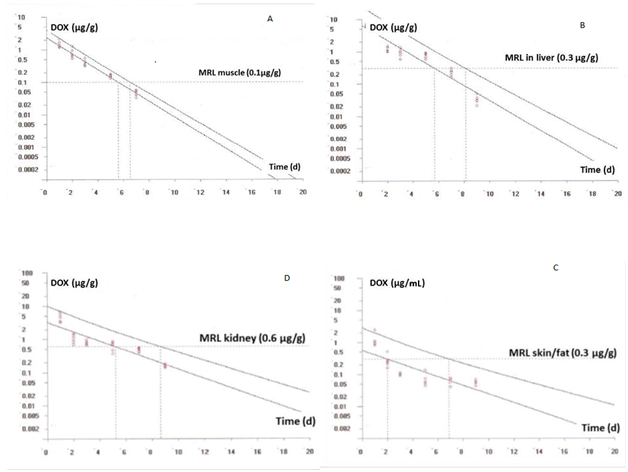

Figure 4 Plot of the withdrawal times calculation for DOX in chicken muscle (A), kidney (B), skin/fat (C) and liver (D) at the time when the one-sided 95% upper tolerance limit is below the EU MRL for doxycycline after its oral administration (5 doses of 10mg kg-1 body weight of DOX 25%). [Residue marker: doxycycline].
In our study, taking into account the MRLs in broilers and considering that the marker residue is the doxycycline, the calculated withdrawal time was 6.58, 8.18, 8.69 and 6.96 d for muscle, liver, kidney and skin/fat, respectively (Figures 4).
In this study chickens were treated with 10mg kg–1 body weight of DOX (25%) once a day, for five consecutive days with drinking water. There are several studies which show data about depletion of DOX from poultry tissues after experimental administration, but some of them used higher doses and different routes of administration compared to our study: Anadón et al.10 published that they treated poultries with 20 mg kg–1 body weight of DOX orally, once a day, for four consecutive days; El–Gendi et al.,16 used a single dose of DOX (20 mg kg–1 body weight) intravenously. On the other hand, Gajda et al.,4 treated animals with the same doses of the present study (10 mg kg–1 body weight of DOX), the same route of administration (orally), once a day, for five consecutive days.
The data obtained in this assay after DOX administration in drinking water demonstrates that, at the beginning after treatment, DOX reached high concentrations in all edible tissues. One day after administration of the last dose, DOX concentration rapidly decreased in all assayed tissues, these results are in concordance with those of other researches despite the doses and the administration route of DOX.10,16,17 Then, the residues decreased gradually and only trace concentrations were observed on day 9.
In this study the highest content of DOX was found in the kidney and liver at one day after treatment was completed. Coinciding with these results, the highest DOX concentrations were observed in kidney and liver by other researchers16–19 but the concentrations reported by Gajda et al.,18 were higher than ours possibly because DOX was administered by syringe into the crop of each chicken.
Other authors16,17 have reported that doxycycline residues are still present in chicken tissues five days after oral administration, and the concentrations are dependent on the applied dose. Other factors that affect the levels of residues can be the administration route, formulation type, excipients and particularly the robustness of the analytical methodology used to quantify the concentrations of the assayed compound.
As final conclusion, results from the present assay demonstrate that DOX oral administration at the dose of 10mg kg–1 for five days in drinking water requires 9 days of withdrawal time for human consumption of these chickens, respecting this way the MRL established by EU.
None.
The author declares that there is no conflict of interests regarding the publication of this manuscript.

©2018 Mestorino, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.