eISSN: 2469-2778


Research Article Volume 12 Issue 3
1Department of Physiology, Faculty of Medicine; Director of Training Department, Research-Consultation &Training Center, Sabratha University, Libya
2Department of biomedicine, Libyan academy of postgraduate studies, Libya
Correspondence: Jbireal JM, Department of Physiology, Faculty of Medicine; Director of Training Department, Research-Consultation &Training Center, Sabratha University, Libya
Received: July 27, 2024 | Published: August 27, 2024
Citation: Jbireal JM, Mohamed BM. The treatment and diagnostic plan for chronic myeloid leukemia in Libyan oncology centers. Hematol Transfus Int. 2024;12(3):64-71. DOI: 10.15406/htij.2024.12.00334
Background: CML is one of the best understood diseases from the aspect of its cytogenetic abnormalities and the molecular mechanisms involved. CML was the first human disease in which a specific abnormality of the karyotype, the Philadelphia (Ph) chromosome, could be linked to a malignant disease. Later on, it was shown that the Ph chromosome results from a reciprocal translocation between the long arms of chromosomes 9 and 22, which produces the BCR-ABL fusion oncogene. Accordingly, in this study; we are going to determine whether the team workers in Libyan oncology centers actually following these steps of guidelines as it has been used internationally or there are some differences and special circumstances prevent them to apply the guidelines.
Objectives: The present study aimed to explore the methods and plans used for diagnosis and treatment of CML patients in Libyan oncology centres and to confirm the most effective methods in diagnosis and treatment of CML by using a statistical analysing of some special data related to a well investigated cases by making a comparison between those plans.
Methods: 173 and 153 CML patient files who has registered in Sabratha oncology institute and Misurata oncology institute -which are both considered as the main oncology centers in Libya- during the last sixteen years were investigated. Then, ten CML case files have randomly chosen from total CML patient files (51) and (78) cases from both oncology institutes which represents (35% and 45%) of all cases respectively (Male and Female, five from each institute). All results of laboratory investigations (CBC, biochemistry profiles, blood film, bone marrow, cytogenetic and molecular analysis) have been recorded and statistically analyzed to know the hematological, cytogenetic and molecular responses during the treatment period. CML patients chosen were at varying disease stages (chronic, accelerated, and blastic phases). The drugs used for treatment included 500 mg of Hydroxyurea, 400 mg, 600 mg and 800 mg of Glivec, and 600 mg of Tasigna in rare cases.
Results and conclusion: The study has indicated the existence of a clear deficiency gap represented in a widespread lack of many capabilities such as the lack of some devices, equipments, operating materials and qualified experts. Moreover, the study has confirmed that the treatment plan has modified depending on the results of the complete blood count (CBC) only during all periods of the treatment without achieving other important investigation used for treatment follow up such as bone marrow aspiration, blood film examination, cytogenetic examination and molecular diagnostic. This study-as the first study dealing with this important topic- clarified many shortcomings of the diagnostic and treatment plan followed in both oncology institutes. As a recommendation, it is necessary to apply all international standard steps of the diagnosis and treatment in particular.
Keywords: chronic myeloid leukemia, ph. chromosome, imatinib mesylate, BCR-ABL1
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm with an incidence of 1–2 cases per 100,000 adults. It accounts for approximately 15% of newly diagnosed cases of leukemia in adults.1 It is a rare disease, yet it has had a profound impact on the development of modern, evidence-based medicine. Additionally, CML was the first human disease in which a specific abnormality of the karyotype, the Philadelphia (ph) chromosome, could be linked to a malignant disease.2
Later on, it was shown that the Ph chromosome results from a reciprocal translocation between the long arms of chromosomes 9 and 22, which produces the Breakpoint Cluster Region-Abelsone murine leukemia viral oncogene (BCR-ABL) fusion oncogene.3 BCRABL is a constitutively active tyrosine kinase, a feature that is critical to the protein’s ability to induce leukemia and provides the rational basis for Abl kinase-targeted therapy of Ph-positive leukemia with ABL kinase inhibitors.4
Currently, it is known that CML can occur at any age, but the incidence greatly increases in the older population due to exposure to ionizing radiation. Weight loss, fever, and abdominal fullness are the main symptoms of this disease. Clinically, 50% of CML cases are asymptomatic. Laboratory findings include a complete blood count, peripheral blood and bone marrow examinations showing low hemoglobin, a total WBC count between 287×109 /L and 535.7×109 /L, thrombocytopenia or normal platelet count or thrombocytosis, and a peripheral blood smear showing an increase in the number of mature and immature granulocytes, including predominantly.5
It is extremely important to know that in the past four decades, there have been outstanding developments in the knowledge and understanding of the genetic and molecular basis of many diseases. In particular, the genetic and molecular basis of the CML have been evaluated.6,7 With regard to CML diagnosis, investigating the presence of a (ph) chromosome abnormality, the t[9; 22] [q34; q11], by routine cytogenetics, or the (ph) related molecular BCR-ABL1 abnormalities by fluorescence in situ hybridization (FISH) or by molecular studies is adequate.8–10 It is extremely important to know that in the past four decades, there have been outstanding developments in the knowledge and understanding of the genetic and molecular basis of many diseases. In particular, the genetic and molecular basis of the CML have been evaluated.6,7. With regard to CML diagnosis, investigating the presence of a (ph) chromosome abnormality, the t[9; 22] [q34; q11], by routine cytogenetics, or the (ph) related molecular BCR-ABL1 abnormalities by fluorescence in situ hybridization (FISH) or by molecular studies is adequate.8–10
Definitely, as a first step in the routine laboratory diagnosis, a blood count is performed under any clinical situation, preoperatively or even at a check-up. Importantly, several methods can be employed for the diagnosis of CML patients, including microscopic examination of peripheral blood and bone marrow, cytogenetics, and molecular biology.11 It is extremely important that CML be differentiated from leukemoid reactions, which usually produce WBC counts lower than 50×109/L, toxic granulocytic vacuolation, Dohle’s bodies, absence of basophilia, and normal or increased leukocyte alkaline phosphatase (LAP) levels. Intrinsically, cytogenetic analysis detects the Ph chromosome in approximately 95% of patients with CML at the time of diagnosis. The rest of the of the CML cases carry masked translocations that can be detected only by molecular techniques, such as FISH or reverse transcriptase-polymerase chain reaction (RT-PCR) for the BCR-ABL fusion.12,13
Accordingly, as an international plan for CML diagnosis, it is very important to apply the following steps.14: medical history and physical exam, including spleen size, complete blood count (CBC) and differential count of leukocytes (DLC), chemistry profile, bone marrow biopsy and aspiration, study of chromosomes by cytogenetic testing, qPCR using international scale (IS) for BCR-APL1 in blood, and hepatitis B. For CML treatment, Since the 2000s, antimetabolites such as hydroxyurea, alkylating agents, and steroids, which were used in the past as a treatment of CML in the chronic phase, have been replaced by BCR-ABL tyrosine-kinase inhibitor [TKI] drugs that specifically target BCR-ABL, the constitutively activated tyrosine kinase fusion protein caused by the Philadelphia chromosome translocation.15 Moreover, standard treatment options for CML patients in the chronic phase before the imatinib era were cytoreductive chemotherapies, INF-α, and allogeneic stem cell transplantation. Chemotherapies, such as hydroxyurea and busulfan, can effectively reduce the tumor burden. However, cytogenetic responses are rare, and these drugs can hardly modify the natural history of CML.16
One of the most important terms used in this context is targeted therapy, which means a drug therapy that focuses on specific, unique features of cancer cells. Targeted therapies seek out how cancer cells grow, divide, and move in the patient's body. Those drugs stop the action of molecules that help cancer cells grow and/or survive.14 The three commercially available TKIs for the frontline treatment of CML include imatinib, dasatinib, and nilotinib. Current guidelines endorse all three as options for the initial management of CML in the chronic phase (CML-CP).17
Imatinib mesylate was the first TKI to receive Food and Drug Administration [FDA] approval for the treatment of patients with CMLCP. It acts via competitive inhibition at the ATP-binding site of the BCR-ABL1 oncoprotein, which results in the inhibition of phosphorylation of proteins involved in cell signal transduction. It also blocks the platelet-derived growth factor receptor and the C-KIT tyrosine kinase.18 In this context, other strategies for frontline therapy include using higher doses of imatinib or combining a TKI with an additional agent, such as IFN-a (Figure 6). In the Tyrosine Kinase Inhibitor Optimization and Selectivity [TOPS] study, patients were randomized to receive imatinib 400 mg once daily or twice daily [800 mg].19 Besides that, the second and third generation TKIs were developed for CML treatment, to overcome imatinib resistance, and to increase responsiveness to TK inhibitors, including four novel agents. Dasatinib is an oral, second-generation TKI that is 350 times more potent than imatinib in vitro.20–22 It also inhibits the Src family of kinases, which may be important in blunting critical cell signaling pathways.23
Moreover, in 2012, radotinib and bosutinib joined the novel agents in inhibiting the BCR-ABL protein. They were used in treating adult CML patients who were Ph chromosome-positive [Ph+] and resistant or intolerant to prior therapy.24 On the other hand, nilotinib is a structural analog of imatinib. Its affinity for the ATP binding site on BCR-ABL1 is 30–50 times greater in vitro.25 It has initially demonstrated the ability to induce hematologic and cytogenetic responses in patients who had failed imatinib. Additionally, with a minimum follow-up of 5 years, the two arms of nilotinib demonstrated better early results compared with imatinib.26
Actually, depending on the experience, we hypothesized in principle that there are some difficulties facing both physicians and patients that prevent them from reaching a definitive diagnosis or effective treatment. Therefore, this study was conducted to determine whether the team workers in Libyan oncology centers are actually following the steps of the guidelines as they have been used internationally, if there are some differences, or if there are some special circumstances that prevent them from applying the guidelines.
Study design and patients: A descriptive, documentary, and screening study has been carried out in Libya. All CML cases in five years (2016–2020) that had been registered in the main oncology centers (Sabratha oncology institute (SOI) and Misurata oncology institute (MOI)) have been collected (Table 1 & Figure 1). Then, 10 CML case files have been randomly chosen from the total CML patient files [78] and [51] from MOI and SOI, respectively.
|
Years |
Total cases (Misurata oncology inst.) in 5 y. |
Total cases (Sabratha oncology inst.) in 5 y. |
|
2016 |
17 |
7 |
|
2017 |
21 |
14 |
|
2018 |
18 |
8 |
|
2019 |
13 |
9 |
|
2020 |
9 |
13 |
|
Total |
78 |
51 |
|
Representative percent |
45% |
35% |
Table 1 Total number of CML cases registered in the last five years in both oncology institutes in Libya
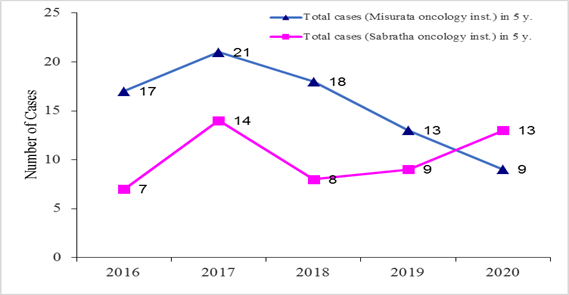
Figure 1 Total number of CML cases registered in the last five years in both oncology institutes in Libya.
After those files had been chosen, all the results of laboratory investigations [CBC, biochemistry profiles, blood film, and bone marrow] had been recorded. Additionally, cytogenetic and molecular analyses have been recorded to determine the hematological, cytogenetic, and molecular responses during the treatment period. The CML patients chosen were at varying disease stages [chronic, accelerated, and blastic phases]. The drugs used for treatment included 500 mg of Hydroxyurea, 400 mg, 600 mg, and 800 mg of Glivec, and 600 mg of Tasigna in rare cases. Ethical approval was obtained from the ethical committee of the Libyan Academy of Science and from the recommendations of each hospital or health center determined as a point for data collection.
In order to follow up on the different stages of treatment and to know the progress of the disease during the treatment period, a random patient file has been chosen to be considered as a typical case [as an example of treated CML patients in Libyan oncology institutes] and to determine the diagnostic and treatment plan followed in these institutes during the same period mentioned above [2016–2021]. Moreover, all data from investigations such as hematological, cytogenetic, and molecular diagnostics have been collected and statistically analyzed by dividing the treatment period into six periods, as will be discussed later.
The results of the BCR-ABL gene have been collected for two patients [one registered in MOI and the other registered in SOI for different periods to be compared to know the effect of the treatment on the expression of this gene by using different doses of imatinib mesylate. Accordingly, a very important fact has been confirmed: both oncology institutes apply different approaches, either for the diagnosis or for the treatment. Statistical analyses: to get into the final conclusion, statistical analysis has been applied to analyze all data supposed to be collected or obtained, and then to make a final comparison using the significant value, statistical package for social science [SPSS] V.21 has been used.
Effect of the treatment of CML on hematological parameters
The mean value of WBCs for CML-diagnosed cases at admission was [157x103] with a standard error [SE] of [±16.3], and then by the time of continuous treatment, the total number of WBCs had been reduced to low numbers with the mean value/SE of [10.5±2.3], 11.2±5.9, 7.6±4.1, 2.4±0.5, and 2.9±0.1 during the period of 4–5 years of treatment, respectively, with a high significant value [P value = 0.0001], which means that the effect of the treatment on the number of WBCs was high (Table 2 & Figure 2).
|
PeriodsParameters |
At admission |
1 Year |
2 Years |
3 Years |
4 Years |
5 Years |
F |
P Value |
|
Mean±SE |
Mean±SE |
Mean±SE |
Mean±SE |
Mean±SE |
Mean±SE |
|||
|
WBCs Count [x103] |
157.7±16.3 |
10.5±2.3 |
11.2±5.9 |
7.6±4.1 |
2.4±0.5 |
2.9±0.1 |
44.418 |
0 |
|
RBCs Count [x106] |
3.53±0.19 |
3.86±0.20 |
3.93±0.23 |
4.04±0.25 |
3.4±0.20 |
3.3±0.10 |
1.166 |
0.344 |
|
Hb [gm/dl] |
9.4±0.54 |
10.5±0.53 |
10.98±0.61 |
11.34±0.58 |
10.93±0.47 |
11.70±0.4 |
1.637 |
0.175 |
|
Platelets Count [x103] |
242±33 |
202±17 |
206±19 |
203±29 |
181±48 |
141±8.5 |
0.82 |
0.545 |
Table 2 Effect of treatment of CML on hematological parameters at different periods
On the other hand, the effect of the treatment on the platelet count [PLTs x103] was slightly high because the mean value of the total number of their count has been reduced, as shown in Table 2, from 242±33 at admission to 141±8.5 at the end of the treatment period, with a low significant value. Conversely, the effect of the treatment on the other main hematological parameters was mild, with a little change within the normal range, especially the effect on RBC count [x106] [3.53±0.19] at admission to 3.3±0.10 at the end of the treatment period and on hemoglobin concentration [g/dl] [9.4±0.54 at admission to 11.70±0.4] with no significant value (Table 2).
Effect of the treatment of CML on the BCR-ABL ratio
As it has been expected, using Imatinib mesylate as the main treatment for CML cases was very effective (Table 3) because the mean value of the BCR-ABL ratio [%] has been significantly reduced from [76.2±8.9] at admission to [0.0±0] after 5 years of treatment (Figure 3).
|
Periods |
At admission |
1 Year |
2 Years |
3 Years |
4 Years |
5 Years |
|
Parameters |
Mean ± SE |
Mean±SE |
Mean±SE |
Mean±SE |
Mean±SE |
Mean ± SE |
|
BCR-ABL |
76.2±8.9 |
36.1±13.6 |
4.4±2.7 |
0.0±0 |
- |
0.0±0 |
Table 3 Effect of Treatment of CML on BCR-ABL at different periods in 10 CML cases that have been chosen and studied randomly
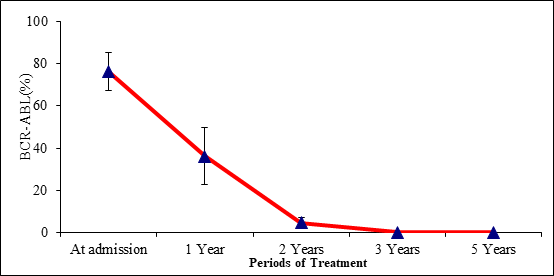
Figure 3 Effect of Treatment of CML on BCR-ABL at different periods in 10 CML cases that have been chosen and studied randomly.
Variance between the mean values of different hematological parameters in different cases due to the effect of CML treatment
Due to the big difference in the mean square values within groups and between groups regarding WBCs (Table 4), this led to the result that [f value] is high, which means that the significance of the difference is very high [P value ˂ 0.0001]. Conversely, with regard to the effect of the treatment on other hematologic parameters such as RBCs, hemoglobin concentration, and platelets, the current study has concluded that the variance was low with no significance [P value ˃ 0.05].
|
Sum of Squares |
df |
Mean Square |
F |
Sig. |
||
|
WBCs |
Between Groups |
170326.972 |
5 |
34065.39 |
44.418 |
0 |
|
Within Groups |
28376.629 |
37 |
766.936 |
|||
|
Total |
198703.601 |
42 |
||||
|
Hb |
Between Groups |
23.084 |
5 |
4.617 |
1.637 |
0.175 |
|
Within Groups |
104.38 |
37 |
2.821 |
|||
|
Total |
127.464 |
42 |
||||
|
RBCs |
Between Groups |
2.361 |
5 |
0.472 |
1.166 |
0.344 |
|
Within Groups |
14.573 |
36 |
0.405 |
|||
|
Total |
16.934 |
41 |
||||
|
PLTs |
Between Groups |
21456.072 |
5 |
4291.214 |
0.82 |
0.545 |
|
Within Groups |
162315.365 |
31 |
5235.98 |
|||
|
Total |
183771.437 |
36 |
Table 4 ANOVA test of 10 cases.
Non-significant difference: [P≥0.05].
Molecular diagnostics and follow-up on the effect of the treatment
In order to confirm the importance of the periodic examination of BCR-ABL percent after each period of treatment, depending on the treatment plan, results of RT-PCR have been collected as a percent of BCR-ABL for two CML cases at different times. Each of them followed a different plan for the treatment depending on the dose concentration and timetable of the treatment. The first case was admitted to the oncology institute [SOI] on October 19, 2016 with a BCR-ABL gene [59.89%], and the protocol for treatment doses was 400 mg IM. This protocol has been continued from the aforementioned date up to January 23, 2019, when the percent of BCR-ABL was reduced to 0.033%, which was a very long period. Subsequently, at the date of February 18, 2019, the patient started taking 300 mg of IM, where the percent of BCR-ABL reached 0.00% (Table 5 & Figure 4).
|
Date |
PCR result |
Treatment dose |
|
19. 10. 2016 |
59. 89 % |
400 mg |
|
23. 1. 2019 |
0.03% |
400 mg |
|
18. 2. 2021 |
0.00% |
300 mg |
Table 5 PCR results of CML patient – [Case study, SOI]
Conversely, the other case was admitted to the oncology institute [MOI] on November 11, 2019, with a BCR-ABL of 53.0%. At that time, the treatment dose was 600 mg IM. The reduction in the percent of BCR-ABL has decreased to 12.4% within about 2 months by using the same dose of IM [600 mg] (Table 6 & Figure 5).
|
Date |
PCR result |
Treatment dose |
|
11. 11. 2019 |
53.00% |
600 mg |
|
18. 12. 2019 |
28.60% |
600 mg |
|
14. 01. 2020 |
12. 4 % |
600 mg |
Table 6 PCR results of CML patient – [Case study, MOI]
Significantly, the statistical analysis of the ANOVA test has confirmed the highly significant difference in the variation between the values of all hematologic parameters [between groups and within groups of data], especially the concentration of hemoglobin [P value = 0.000], which was very high.
Similarly, the variance in the case of the total number of WBCs was high too because of the value of degree of freedom [df = 5] and the value of F = 4.610, which led to a high significant difference [P value = 0.004], whereas the significant difference in the case of the PLT count was slightly high [P value = 0.016] (Table 7).
|
Sum of Squares |
df |
Mean Square |
F |
Sig. |
||
|
WBCs |
Between Groups |
4811.794 |
5 |
962.359 |
4.61 |
0.004 |
|
Within Groups |
5010.636 |
24 |
208.776 |
|||
|
Total |
9822.43 |
29 |
||||
|
Hb |
Between Groups |
59.95 |
5 |
11.99 |
23.698 |
0 |
|
Within Groups |
12.649 |
25 |
0.506 |
|||
|
Total |
72.599 |
30 |
||||
|
PLT |
Between Groups |
14909.374 |
5 |
2981.875 |
3.502 |
0.016 |
|
Within Groups |
21284.045 |
25 |
851.362 |
|||
|
Total |
36193.419 |
30 |
Table 7 ANOVA test of the case study
[P value ˂ 0.05]
The data in Table 8 show the correlation between the variation of hematological parameters [WBCs, Hb. Conc., and PLTs count] as compared with the different periods of treatment. This correlation appeared as a significant negative correlation between the treatment periods [time of treatment] and the total number of WBCs[-0.829], which means the opposite relation between them; the longer the treatment period, the lower the WBC count [P value = 0.042].
|
Spearman's Correlation |
Periods |
WBCs |
Hb |
PLTs |
|
|
Periods |
Correlation Coefficient [r] |
- |
-0.829* |
0.429 |
0.086 |
|
Sig. [2-tailed] |
. |
0.042 |
0.397 |
0.872 |
|
|
WBCs |
Correlation Coefficient [r] |
-0.829* |
- |
-0.257 |
-0.086 |
|
Sig. [2-tailed] |
0.042 |
- |
0.623 |
0.872 |
|
|
Hb |
Correlation Coefficient [r] |
0.429 |
-0.257 |
- |
0.886* |
|
Sig. [2-tailed] |
0.397 |
0.623 |
- |
0.019 |
|
|
PLTs |
Correlation Coefficient [r] |
0.086 |
-0.086 |
0.886* |
- |
|
Sig. [2-tailed] |
0.872 |
0.872 |
0.019 |
- |
|
Table 8 Correlation between hematological parameters and periods of treatment [case study, SOI]
Equally, there was a positive correlation between hemoglobin concentration and PLT count during different treatment periods [r = 0.886] with a significant value [P value = 0.019]. Remarkably, there was no positive or negative correlation between doses of the treatment and the changes in the values of hematologic parameters during different periods of the treatment; therefore, no significant difference has been detected.
Chronic myeloid leukemia (CML) is a clonal malignant neoplasm of pluripotent hematopoietic stem cells characterized by the excessive proliferation of mature granulocytes and their precursors in the bone marrow and peripheral blood, caused in 90% of cases by the presence of the Philadelphia chromosome and rarely by hyperdiploidy of >50 chromosomes.27 As mentioned above, the course of the disease is characteristically triphasic: a chronic phase (CP) lasting three to six years is followed by transformation to an accelerated phase [AP] and then a terminal blast phase of short duration.7,20
In 2017, it has been estimated that about 9000 new CML cases will be diagnosed in the United States, and about 1000 patients will die of CML. Since the introduction of imatinib in 2000, the annual mortality in CML has decreased from 10%–20% down to 1%–2%.1 It goes without question that periodic investigation results tell the physicians how to modify the treatment plan. It is important to understand what these investigations mean and whether they should go to a second opinion, investigation, or office visit.
Unfortunately, as we have seen in the last chapter of the results, the treatment plan was modified depending on the results of the complete blood count [CBC] only during all periods of the treatment without achieving other important investigations used for treatment follow-up, such as bone marrow aspiration, blood film examination, cytogenetic examination, and molecular diagnostics. Those investigations are known as a first line and a routine procedure according to the international plan.14
One of the most important facts in this context is that using IM as a treatment for CML patients may lead to the phenomenon called [ematinib resistance]. The mechanisms of imatinib resistance were first studied in cell culture-based systems. BCR-ABL-positive leukemic cells cultured in suboptimal concentrations of imatinib for prolonged periods of time developed moderate imatinib resistance to concentrations of up to approximately 1 mM.28–30
As it has been confirmed according to the results mentioned in the last chapter in the context of the case study, treating the patient with IM has taken a long time [2016–2019] to reach the ratio of 0.00% of BCR-ABL. Clinically, primary resistance describes the failure to obtain a sufficient response despite adequate imatinib treatment. Primary hematologic resistance can be assumed if a patient does not achieve any hematologic response after three months or no complete hematologic response after six months, despite sufficient imatinib treatment.
Conversely, about the other case [MOI] who admitted to the oncology institute on November 11, 2019, it was clear that using 600 mg IM for about 2 months, the molecular response [BCR-ABL ratio] has clearly reduced to a low level [from 53.0% to 12.4%] (Figure 5). whereas the other case, who admitted to the oncology institute on October 19, 2016 with a 59.89% BCR-ABL ratio, took more than 2 years to reach a percent of 0.033% and another 2 years [from January 23, 2019 to April 18, 2021] to detect that the ratio of BCR-ABL had reached 0.00% by using 400 mg IM during the first period, then the dose was reduced to 300 mg with no detection of the molecular response through that period (Figure 4).
Case studies illustrating the importance of molecular monitoring
The current study detected that most of the CML cases in both oncology institutes were treated with IM without monitoring the BCR-ABL ratio during the treatment period, which was in contrast with the international plan, which prolonged the treatment period for most of the cases.
This argument is consistent with what has been proven by numerous studies that demonstrated the importance of monitoring patients using reliable and reproducible RQ-PCR for detecting biologically significant changes. In one of those studies, patient A commenced imatinib 400 mg daily in the chronic phase and achieved a CCyR. A more than two-fold rise in BCR-ABL prompted mutation analysis, and the non-P-loop F317L mutation was identified. This mutation confers moderate resistance to imatinib. The imatinib dose has been increased to 600 mg daily, and the patient has again achieved sustained CCyR. Patient B commenced imatinib with a fluctuating dose of 400 to 600 mg. Molecular analysis was not performed until six months, and it is unknown if a significant rise in BCR-ABL levels occurred prior to the detection of the F317L mutation. The imatinib dose was increased to 800 mg. The highly imatinib-resistant P-loop mutation Y253H became detectable, and the BCR-ABL level began to rise over the following six months. The Y253H mutation is known to be sensitive to dasatinib.23
It is worth mentioning here that the investigation of cytogenetics, bone marrow analysis, and fluorescence in situ hybridization can be used to detect minimal residual disease in patients in cytogenetic remission, but it is not as sensitive as RQ-PCR and requires cells to be in metaphase.31 Accordingly, because the cytogenetic response correlates well with the duration of treatment,5 we think that the treatment strategy used in both oncology institutes lacks precision as it is largely randomized and more experimental than following a specific treatment plan. This proves the fact that the treatment strategy must be modified to meet the international standards required to treat such cases. Currently, data and results collected from different studies strongly suggest that intrinsic sensitivity to imatinib is variable in previously untreated patients with CML, and the level of Bcr-Abl kinase inhibition achieved is critical to the imatinib response.
High-dose Imatinib and Imatinib-based combinations
Regardless of the aforementioned, the purpose of implementing an accurate treatment plan according to international standards is to reach the highest degree of efficacy, so it is necessary to apply all international standard steps of diagnosis and treatment in particular. Therefore, during the conduct of this study, we did not notice any use of innovative treatment plans, as stated by many important studies in this field.
One of those efforts is to use high-dose IM and an IM-based combination. Those include using higher doses of imatinib or combining a TKI with an additional agent, such as IFN-a. The current study has confirmed that the treatment plan followed in both oncology institutes was depending on using different doses of IM in low concentrations ranging between 200 mg and 600 mg over a long period of time [Case study result chapter], with those doses changing from time to time randomly according to the results of CBC.
Finally, this study—as the first study dealing with this important topic—clarified many shortcomings of the diagnostic and treatment plans followed in both oncology institutes. One of the most important things that should be done periodically is that all patients should undergo a bone marrow examination to establish the diagnosis, assess the percentage of blasts and basophils, and perform cytogenetic analysis to confirm the presence of the Philadelphia chromosome and to exclude clonal evolution, particularly [i17,.q10], 27/del7q, and 3q26.2 rearrangements, associated with a relatively poor prognosis.32 The current recommendation that patients have a follow-up bone marrow study at 3, 6, and 12 months after starting therapy may no longer be necessary.33
As a conclusion, depending on the aforementioned objectives, this study has revealed many important facts with regard to the diagnostic process and the treatment plan followed in both oncology institutes in Libya. Regardless of whether the physicians are applying the right diagnostic procedures or an effective treatment plan, the reality of the health situation indicates the existence of a clear deficiency gap represented by a widespread lack of many capabilities, such as the lack of some devices, equipment, operating materials, and qualified experts. Additionally, this study indicated that the treatment plan has been modified depending on the results of the [CBC] only during all periods of the treatment without achieving other important investigations used for treatment follow-up, such as bone marrow aspiration, blood film examination, cytogenetic examination, and molecular diagnostics. This study, as the first to deal with this important topic, clarified many shortcomings of the diagnostic and treatment plans followed in both oncology institutes; therefore, as a recommendation, it is necessary to apply all international standard steps of diagnosis and treatment in particular.
None.
The author declares that there are no conflicts of interest.

©2024 Jbireal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.