eISSN: 2469-2778


Case Report Volume 7 Issue 2
1Department of Internal Medicine, Reading Hospital, USA
2Department of Internal Medicine, Jersey City Medical Center, USA
3Department of Rheumatology, Jersey City Medical Center, USA
Correspondence: Murad Baba, Department of Internal Medicine, Reading Hospital, USA
Received: March 07, 2019 | Published: March 15, 2019
Citation: Baba M, Dominguez L, Marian V. The conundrum of cardiac sarcoidosis. Hematol Transfus Int J. 2019;7(2):22-25. DOI: 10.15406/htij.2019.07.00199
Introduction: Sarcoidosis can be a benign, incidentally discovered condition or a life-threatening disorder causing sudden death. The frequency of myocardial involvement is unclear; small registries suggest 5 percent of patients with systemic sarcoidosis, while autopsy studies suggest subclinical cardiac involvement in up to 70 percent. The range of sarcoid involvement of the heart includes heart block and arrhythmias, heart failure, valvular dysfunction, simulated infarction, and pericardial disease.
Case Description: 37 year-old African American female with past medical history of presumed asthma presented to the emergency room complaining of progressively worsening dyspnea, chest pain and lightheadedness. Physical examination showed tachycardia with displaced point of maximal impulse (PMI) and minimal rhonchi on right lung base. Laboratory workup did not show any significant results on presentation. Chest X-ray showed bilateral interstitial infiltrates and the chest Computed Tomography (CT) Angiogram showed bilateral scattered pulmonary nodules, right-sided pleural effusion with thickening. Lung biopsy showed non-caseating granulomas typical of sarcoidosis. Further workup revealed Ejection Fraction (EF) of 25% on Transthoracic Echocardiogram (TTE), cardiac catheterization showed normal coronaries and electrophysiological study for inducible arrhythmias was negative.
Conclusion: Cardiac sarcoidosis may be suddenly fatal. Therefore, clinicians must maintain a high degree of suspicion for cardiac involvement and screen all patients with extra cardiac sarcoidosis. Despite significant advances in immunosuppressant pharmacotherapy, the backbone of therapy for cardiac sarcoidosis remains systemic corticosteroids.
Keywords: cardiac sarcoidosis, cardiac MRI, sarcoidosis, pleural effusion
Sarcoidosis is a systemic disease, defined by non-caseating granulomas, which invade and affect the function of one or more organs.1 It has both environmental and genetic components. Most recently, sarcoidosis has been found to be epidemic among firefighters.2 Other investigations have found that sarcoid has a hereditary component.3 However, not all cases can be traced back to environmental exposures or genetic inheritance. Sarcoidosis is not a common disease, and its prevalence follows certain demographics. The incidence of sarcoidosis in the general population is 10.9/100,000 people.4 However, sarcoidosis disproportionately affects African Americans, with an incidence rate of 35.5/100,000.4
37 year-old African American female with past medical history of presumed asthma presented to the emergency room complaining of progressively worsening dyspnea that was associated with pleuritic chest pain and cough productive of yellowish-greenish sputum. She also had a syncopal episode just prior to arrival to the hospital. Her dyspnea was exertional and progressing over the last few months with orthopnea and paroxysmal nocturnal dyspnea (PND).
Physical Examination revealed tachycardia with displaced point of maximal impulse (PMI) and minimal rhonchi on right lung base. Laboratory workup did not show any significant results on presentation. Chest X-ray showed bilateral interstitial infiltrates with areas of fibrosis and the chest CT Angiogram (Figure 1) showed bilateral scattered pulmonary nodules and densities, a right-sided pleural effusion, pleural thickening and cardiomegaly.
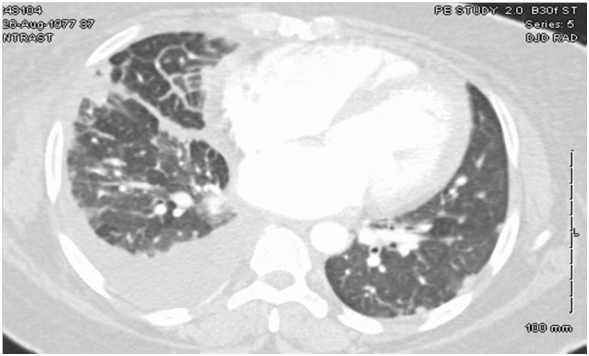
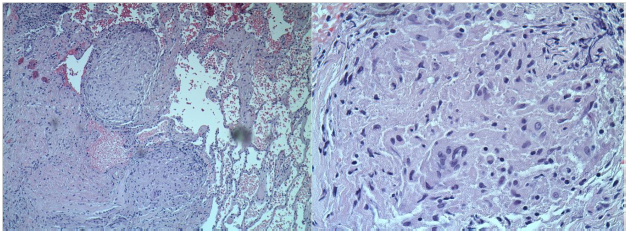
TTE showed EF of 25% with type III advanced diastolic dysfunction, further cardiac workup was done. This included cardiac catheterization which showed normal coronaries and an electrophysiological study for inducible arrhythmias which was negative. She was offered a lung biopsy; the pathology revealed classic non-caseating granulomas as shown in Figure 2.
The patient was referred for a cardiac magnetic resonance imaging (MRI), which showed Left Ventricle (LV) dilation and severe LV dysfunction. The contrast study showed diffuse contrast late enhancement with patchy and confluent patterns characteristic of myocardial infiltrative process Figure 3.
After starting systemic corticosteroids she improved significantly and was discharged home with a life vest and optimized on Congestive Heart Failure (CHF) medical therapy. She was also advised for Implantable Cardioverter Defibrillator (ICD) but refused. TTE was repeated six months following systemic corticosteroid therapy and showed significant improvement in EF from <25% to 40%.
Clinical presentation
While pulmonary involvement is the hallmark, cardiac manifestations are present in 20-30% of cases.5 Notably, such involvement is usually asymptomatic, and only uncovered and therefore estimated, based on autopsy.6–8 Autopsy studies suggest subclinical cardiac involvement in up to 70%. Indeed, symptomatic cardiac sarcoid occurs in only 5% of cases.6
Cardiac sarcoidosis can be a benign, incidentally discovered condition or a life-threatening disorder causing sudden death. Patients may be asymptomatic or may report palpitations, syncope, dizziness, or chest pain. An abnormal electrocardiogram (ECG) and cardiac related symptoms at disease onset are important predictors of a later diagnosis of clinically significant cardiac sarcoidosis. In contrast, cardiac sarcoidosis is very rare in subjects without symptoms and with a normal ECG.
When sarcoidosis spreads to the myocardium, it can manifest in a number of ways. Cardiac involvement can cause arrythmogenic, myopathic, valvular, pericardial,9 or coronary artery complications.10 Accordingly, cardiac sarcoidosis can manifest in a multitude of ways, from Atrioventricular block, to dilated cardiomyopathy, pericardial effusions, to coronary ischemia. Regardless of the presentation, cardiac involvement carries a poor prognosis. About 80% of deaths among patients diagnosed with sarcoidosis have had some degree of cardiac involvement. The most common causes of death among patients with cardiac involvement are heart failure or sudden-cardiac-death.11–21 Given the importance of identifying cardiac involvement, unfortunately, the diagnosis of cardiac sarcoid presents many challenges.
Pleural effusions are a rare complication of sarcoidosis. Soskel and Sharma21 estimate that at most, 10% of cases have pleural involvement. Our patient presented with unilateral pleural effusion and cardiac involvement. While the presenting complaint was dyspnea, it is difficult to determine whether the pulmonary or cardiac involvement was the culprit.
Diagnosis
Somewhere between 24-77% of cardiac cases are detectable by TTE. 12 Diastolic failure is one of the earliest signs of sarcoid invasion of the heart 12. While a biopsy of a non-caseating granuloma is near pathognomonic,13 the sensitivity is extremely poor–only 20%, making biopsies a poor diagnostic choice.14 Furthermore, given the natural history of granulomas, while early stages may be identified by inflammation, in later stages, the granuloma might have resolved into a scar or gliosis, making it indistinguishable from other insults.15
Multiple imaging modalities have been attempted in diagnosing cardiac sarcoidosis. The best current scans are cardiac MRI and PET. Cardiac MRI has the greatest capacity to pick up focal myocardial edema indicative of active granulomas, in addition to the scarring left by the old granulomas.16 PET is also extremely sensitive in picking up small amounts of inflammation, caused by granulomas.17
The Japanese came out with rigorous guidelines in 2006 to aid in the diagnosis. They proposed that in the likely event that histology does not provide sufficient answers, the diagnosis can be made clinically. Diagnosis must satisfy either 2 major, or 1 major and 2 minor criteria. The major criteria are Atrioventricular (AV) block, ventricular septal thickening, increasing patchy gallium uptake on cardiac MRI, or an EF of less than 50%. The minor criteria include ECG abnormalities (multi-focal or frequent premature ventricular contractions (PVC), right bundle branch block (RBBB), ventricular arrhythmias or 3rd degree AV block). Other minor criteria include abnormal wall motion, abnormal echocardiography, perfusion defects, delayed enhancement of myocardium on MRI, or infiltration by monocytes and concurrent fibrosis on biopsy.18
Treatment
The treatment for cardiac sarcoidosis is systemic corticosteroids. As cardiac sarcoid is a poor prognostic indicator to begin with, therefore prompt treatment is essential. Waiting for drops in EF or AV blocks can be catastrophic, as by that point, the myocardium has already been damaged, remodeled, and irreparably scarred.19 Indeed, heart failure is one of the most dangerous complications from cardiac sarcoid. The purpose of corticosteroids administration is to prevent such remodeling and gliosis from taking place. Corticosteroids should be administered in doses of 1mg/kg/day, and then tapered after 2-3months, to 10-20mg every other day.20–28
ICD placement should be strongly considered in this population, especially in patients with evidence of extensive myocardial damage and fibrosis or in the presence of arrhythmias, as studies have shown an increased rate of adverse events and sudden cardiac death. As such, placement of an ICD for primary prevention is a Class IIa recommendation.
Cardiac involvement may be the initial manifestation of sarcoidosis and the diagnosis can be challenging. Unlike sarcoidosis involvement of most other organs, it may be suddenly fatal. Therefore, it is important to screen for cardiac sarcoidosis in all sarcoidosis patients. Corticosteroid therapy, although untested in randomized clinical trial, seem to be beneficial particularly when used relatively early in the course of the disease.28 Despite significant advances in immunosuppressant pharmacotherapy, the backbone of therapy for cardiac sarcoidosis remains systemic corticosteroids. Careful follow-up is mandatory when using high dose corticosteroids. In those patients with severe heart failure and minimal extra cardiac sarcoid, consideration should be given for cardiac transplantation.28 This case stands as both an atypical and intriguing presentation of an uncommon disease, illustrating the importance of early intervention and the need for continued development of treatment options for sarcoidosis, a systemic disease with considerable morbidity.
None.
The authors have no conflicts of interest to declare relevant to this article.

©2019 Baba, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.