eISSN: 2469-2778


Case Report Volume 12 Issue 4
1Department Internal Medicine, Medical Sur Hospital, Mexico
2Hematologist, Department of Internal Medicine, Medical Sur Hospital, Mexico
3Department of Pathological Anatomy, Medical Sur Hospital, Mexico
4Dermatologist, Department of Internal Medicine, Medical Sur Hospital, Mexico
5Oncologist, Department of Internal Medicine, Medical Sur Hospital, Mexico
Correspondence: Santiago-Benítez Mary Jose, Department Internal Medicine, Medical Sur Hospital, Mexico, Tel +52922210023
Received: November 24, 2024 | Published: December 3, 2024
Citation: Jose SBM, Omar LN, Felipe ARL. Stevens-Johnson Syndrome secondary to nivolumab in a patient with mixed cell variety classical Hodgkin Lymphoma. Hematol Transfus Int. 2024;12(4):86‒90. DOI: 10.15406/htij.2024.12.00339
The immune checkpoint inhibitors (ICIs) nivolumab and pembrolizumab are some of the principal treatment options for relapsed or refractory classic Hodgkin lymphoma. However, immunotherapy has been related to dermatological adverse events (DAEs) and could lead to discontinuation of cancer treatment in severe cases. DAEs encompass entities such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), SJS/TEN-like reactions, drug reaction with eosinophilia and systemic symptoms (DRESS). We reported a case of a 46-year-old- female with classic mixed cellularity Hodgkin lymphoma, Lugano IV, who underwent a hematopoietic cell transplant and subsequent relapse, currently with Stevens-Johnson Syndrome secondary to nivolumab.
Keywords: Cutaneous toxicity, oncodermatology, toxicities from immunotherapy, Stevens-Johnson Syndrome, nivolumab
ICIs, immune checkpoint inhibitors; DAEs, dermatological adverse events; SJS, Stevens-Johnson syndrome, TEN, toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; CHL, classic hodgkin lymphoma; NLPHL, nodular lymphocyte-predominant HL; HL, hodgkin lymphoma; EBV, epsteir-barr virus; irAEs, immune-related adverse events; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand 1; PIRME, Progressive immunotherapy-related mucocutaneous eruption; CTLA-4, cytotoxic T-lymphocyte-associated protein 4
Hodgkin lymphoma (HL) is a B-cell lymphoid malignancy involving lymph nodes and the lymphatic system. The WHO classification divides HL into two main types: Classic Hodgkin Lymphoma (CHL) and nodular lymphocyte-predominant HL (NLPHL). CHL is divided into four subtypes: nodular sclerosis CHL, mixed cellularity CHL, lymphocyte-depleted CHL, and lymphocyte-rich CHL.1,2 Most patients are diagnosed with HL between 15 and 30 years of age, followed by another peak in adults aged >55 years. Although the exact etiology is unknown, some risk factors for HL include prior infection with Epstein-Barr virus (EBV) and immunocompromising conditions, including immunosuppression after organ transplantation or infection with HIV.3,4 The Lugano classification, currently used for staging, is a modified version of the old Ann Arbor system. Staging is generally defined as I to IV, where stage IV is a disseminated involvement of one or more extra lymphatic organs (e.g., lung, bone marrow, liver) with or without any nodal involvement.5 Classic Hodgkin lymphoma is potentially curable with multimodality treatment, including multiagent chemotherapy and/or radiotherapy. However, relapse can occur in up to 30% of patients with advanced-stage classic Hodgkin lymphoma. The immune checkpoint inhibitors (ICIs) nivolumab and pembrolizumab are some of the principal treatment options for relapsed or refractory classic Hodgkin lymphoma. In patients who decline autologous stem-cell transplantation or who are unsuited for high-dose chemotherapy and subsequent autologous stem-cell transplantation because of comorbidities, the use of ICIs may improve overall survival.6,7 However, immunotherapy has been related to dermatological adverse events (DAEs) and could lead to discontinuation of cancer treatment in severe cases. DAEs encompass entities such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), SJS/TEN-like reactions, drug reactions with eosinophilia, and systemic symptoms (DRESS). We reported a case of a female with classic mixed cellularity Hodgkin lymphoma, Lugano IV, who underwent a hematopoietic cell transplant and subsequent relapse, currently with Stevens-Johnson Syndrome secondary to nivolumab.
A 46-year-old female patient diagnosed with classic mixed cellularity Hodgkin lymphoma, Lugano IV, who underwent a hematopoietic cell transplant in October 2022, with subsequent relapse in April 2023, Whole-body 18FDG-PET/CT (18-Fluorodeoxyglucose-Positron Emission Tomography/computed tomography) activity was reported in cervical lymphadenopathy, axillary, and the internal mammary chain and splenic hilum. Immunotherapy with Nivolumab was started in May 2023. She went to the emergency department due to disseminated dermatitis with erythematous macules, papules, and vesicles on an erythematous base on the anterior and posterior thorax, face, neck, both arms, and proximal region of both lower extremities, which spared the palms and soles, pruritic, with centripetal predominance, morbilliform, confluent, scaly, whitening under digital pressure, covering <10% of the total body surface, (Figure 1) as well as painful, burning oral ulcers in the oral cavity at the level of the soft palate, tongue, and cheeks, bleeding at the mouth opening (Figure 2) feverish peak of 38°C accompanied by conjunctival injection, bilateral non-purulent ocular discharge, decreased acuity visual sensation, sensation of a foreign body, difficulty opening eyelids, periorbital edema, pain in the oral cavity that made swallowing impossible (Figure 3). Upon admission, laboratories showed normocytic normochromic anemia (8.9 mg/dL), HCM 30.1 (pg), VGM 91.3 (fL), severe thrombocytopenia (13 x10∧3/uL), lymphopenia (0.3 x10∧3/uL), elevation of inflammatory markers (C Reactive Protein 57.1 mg/L), procalcitonin (0.29 ng/mL). A chest CT scan was performed with a negative report for lesions due to a secondary deposit and inflammatory reagent, bulla, in the right lower posterior segment and splenomegaly. Management was started with dexamethasone 40 mg every 24 hours and acyclovir 400 mg empirically.
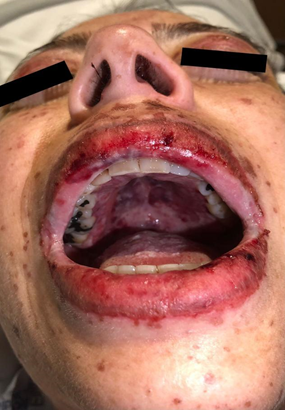
Figure 2 Stevens-Johnson syndrome. Periorbital edema and erythema of both eyes, oral mucosal involvement with wet purple and erosions on lips

Figure 3 Stevens-Johnson syndrome. Erythema of both eyes. Reddish maculopapular lesions on the face, perioral area and neck
An ophthalmology consultation was carried out, a corneal ulcer in the right eye was diagnosed, so treatment with valganciclovir and ophthalmic moxifloxacin was started. Negative serologies were collected for Epstein Barr virus (EBV), Cytomegalovirus (CMV), herpes zoster, and herpes simplex 1 and 2, as well as negative respiratory biofire Polymerase chain reaction. Blood and urine cultures were negative, for which antiviral medication was suspended, and the ocular regimen was changed. Subsequently, she developed dysuria, skin lesions in the vulvar region compatible with lichen planus, and fusion of the labia majora; she was evaluated by gynecology, who performed cervical dilations and management with topical hydrocortisone. Due to oral intolerance, she was maintained on parenteral nutrition. A skin biopsy was performed due to suspicion of pharmacodermy secondary to nivolumab and methylprednisolone was started at 2 mg/kg/day empirically for 7 days. Histopathological report of a subepidermal blister associated with full-thickness epidermal necrosis and apoptotic bodies with perivascular dermal lymphoplasmacytic infiltrate compatible with Stevens-Johnson Syndrome Figure 4A & 4B. A scorten of 2 points was calculated with a mortality of 12% in the acute phase.
Skin toxicities are the most reported adverse effects secondary to immunotherapy (>50% for all grades) but are rarely severe and usually do not impair treatment continuation.8,9 Immune-related adverse events (irAEs) are more common, up to 90% of patients treated with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors, 70% of patients treated with cell death programmed cell death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors, with almost all patients receiving combination therapies.10 Skin reactions to immune control inhibitors account for approximately 40% of their mild form. There is diversity in terms of lesions that range from morbilliform, lichenoid, granulomatous, and psoriasiform reactions, as well as vitiligo-type lesions, eczema, lupus erythematosus, blistering diseases, fortunately, less than 1% correspond to serious reactions during therapy anti-PD1. The relative risk of developing cutaneous adverse effects with nivolumab is 2.3.8 Pruritic and maculopapular rashes are the most frequently associated with immune checkpoint inhibitors (ICIs). Presentation time can occur after a few days of treatment lasting up to 16-20 weeks. There are few cases reported in the literature regarding severe cutaneous adverse reactions secondary to anti-PD1 therapy, so there is a limitation regarding optimal treatment. The study of more cases will help to elucidate the immunological impact, pathogenesis, and optimal treatment strategies, which is why we emphasize the importance of its scientific dissemination.11–14
There are also rare, isolated reports of lethal or potentially lethal adverse events (AEs), such as toxic necrolysis (Lyell syndrome), severe Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), SJS/TEN-like reactions, drug reactions with eosinophilia and systemic syndrome (DRESS), neutrophilic drug eruptions including acute generalized exanthematous pustulosis, cutaneous small-vessel vasculitis and neutrophilic dermatoses (Sweet syndrome and pyoderma gangrenosum-like ulcers).10,15 SJS is a severe adverse mucocutaneous reaction, which is in the spectrum of a pharmacological response with mortality of up to 35%. It covers <10% of the body surface area (BSA), and it usually presents with fever, blisters, erosions, generalized erythema, and mucosal rash, it is frequently caused by medications since 200 types of related drug reactions have been reported to SJS, however, it is not the only cause.16-18 The exact mechanism of damage in the pathophysiology is unknown, however, the theory is recognized as the type IV delayed hypersensitivity reaction mediated by T cells. ICIs interfere with the physiological mechanisms of homeostasis and peripheral immune tolerance, generating a subsequent inflammatory effect, which ends in adverse events related to the immune system. It is known that they can affect any organ system. Unlike classic drugs associated with SJS and NET that reach pharmacokinetic levels in hours, nivolumab reaches a steady state in week 12 of biweekly infusions and has a half-life of 25 days.19
In a retrospective analysis of 98 patients with dermatological lesions secondary to immunotherapy, the predominant morphology described was: Lichenoid, maculopapular, psoriasiform, eczematous, and immunobullous, 24.3% required interruption of immunotherapy. A new term has been proposed for atypical skin lesions similar to those of SJS/TEN: Progressive immunotherapy-related mucocutaneous eruption (PIRME), which, unlike the typical clinical picture of SJS such as the present in which the lesions occurred after the first cycle of nivolumab and a probable second identifiable trigger was not identified, in the atypical lesions there are key findings for differentiation such as little ocular involvement, a favorable response to treatment generally at 72 hours, so it is assumed a course of the mild disease and the involvement of a second triggering drug that, if withdrawn and observed clinical improvement, could give the patient the possibility of continuing treatment with ICIs, without significantly altering the course of treatment against the disease. Biomarkers such as granulysin, a cytotoxic mediator responsible for keratinocyte death, are highly expressed in the blister fluid and serum of SJS/TEN patients. Its concentration correlates linearly with the extent of body surface area involvement. Serum granulysin demonstrates 80% sensitivity and 95.7% specificity for early diagnosis of SJS/TEN in patients with nonspecific drug rashes. Additionally, a rapid immunochromatographic test strip for granulysin has been developed, though it is not yet widely accessible. Granulysin levels can also predict the onset of SJS/TEN 2 to 4 days before skin detachment or mucosal lesion development (p < 0.010).20,21
Diagnosis requires a clinical histopathological correlation with a skin biopsy. Pathological examination of maculopapular rashes shows lymphocytic CD4þ infiltrates with eosinophils and papillary edema.22 Neoplastic disease, especially when we know that there are few or in some cases no subsequent treatment alternatives since current National Comprehensive Cancer Network guidelines indicate permanent discontinuation of ICIs after a serious adverse reaction. There is little information in the literature and there are isolated reports on this phenomenon; however, it is essential to take it into account given that it can impact a patient's prognosis by considering the key characteristics regarding the course of this type of severe cutaneous adverse reactions to checkpoint inhibitors.23 In a cohort conducted in Taiwan between 2008 and 2019, patients with SJS/TEN were also observed to have an average loss of life expectancy of approximately nine years.24 Regarding treatment, since the majority will be mild reactions, approximately 90% will improve with topical corticosteroids.25 However, in severe reactions such as SJS, there are different treatment modalities, such as intravenous boluses of methylprednisolone at 1 gram/kilogram of weight, analgesic support, intravenous hydration, multidisciplinary support with oncology, dermatology, ophthalmology, and gynecology.26 The optimal duration of steroid treatment remains debated; some authors recommend progressively de-escalating it, with a total duration of 4 weeks. The combination of steroids and IV immunoglobulin seems to have good results.27,28
The patient must be in a burn center or in an intensive care unit due to the multi-organ support required. An effort should be made to identify the triggering drug and withdraw it. Supportive care continues to be the cornerstone of treatment for SJS/TEN, as robust evidence for any single therapeutic intervention is limited. Comprehensive supportive care is essential in the early management of SJS/TEN. This approach encompasses ocular, oral, and wound care, as well as genitourinary support, pain control, fluid and electrolyte balance, airway stabilization, stress ulcer prevention, nutritional support, thromboprophylaxis, and antibiotic therapy when there is a confirmed infection; antibiotic prophylaxis is not indicated.29 Cyclosporine, TNF inhibitors like etanercept, and combinations of various agents have shown potential in small observational studies and unblinded randomized controlled trials.30 In recent years, the role of cyclosporine in this type of reaction has been studied, with some reports limiting progression for its immunomodulatory properties; however, more randomized clinical trials are needed.27,31 Recombinant human tumor necrosis factor receptor-Fc fusion protein antibodies have also been used with good outcomes in 3-month follow-up.28 In one study, etanercept showed a reduction in disease-specific mortality. The relative risk (RR) was 0.51 (95% CI 0.16 to 1.63), suggesting a possible reduction in deaths compared with corticosteroids. However, the confidence intervals include both benefits and harms, implying low certainty of the evidence. Serious adverse events were reported in the etanercept group; 5 of 48 participants experienced serious events such as sepsis and respiratory failure, while in the corticosteroid group, this occurred in 9 of 43 cases.
However, it was not specified whether these events led to discontinuation of treatment, and metrics such as time to complete re-epithelialization, length of hospitalization, or ICU stay were not reported.32 In a meta-analysis evaluating the efficacy of three TNF-α inhibitors (etanercept, infliximab, and adalimumab) in terms of mortality, hospitalization time, and re-epithelialization, etanercept was found to be the most promising biologic treatment for SJS/TEN, with lower mortality rates and shorter hospitalization times compared to other therapies, and fewer side effects and sequelae (6.4%) compared to infliximab (39.3%), reinforcing its utility as a primary therapeutic option. Although infliximab had comparable performance in terms of re-epithelialization and hospitalization time, its use was associated with more adverse effects. In patients with superimposed SJS-TEN, etanercept showed a mortality of 0%, compared to 33.3% for infliximab. When subgroup analysis was performed, early administration (within the first 7 days of symptom onset) of etanercept resulted in a significant reduction in mortality. The use of combination therapies (etanercept with corticosteroids and immunoglobulin) increased hospitalization times and the incidence of sequelae compared with etanercept monotherapy.33 In a retrospective, multicenter study of 64 patients, IV immunoglobulin and cyclosporine were compared. The average dose of IVIg was 1 g/kg/day for 3 days, whereas the dose of cyclosporine ranged from 3 to 5 mg/kg/day orally or intravenously for an average of 7 days. Cyclosporine showed a mortality benefit, with a standardized mortality ratio (SMR) of 0.43, indicating lower mortality than predicted. In contrast, IVIg had an SMR of 1.43, suggesting higher mortality than expected in this group of patients. However, the authors note that the doses and timing of the treatments were not uniform, and the heterogeneity of the patient's comorbid conditions could have affected the results.28 There is a need for multicenter randomized controlled trials to better validate the results of these drugs, since most come from reports of isolated clinical cases or small series of cases. However, we understand that severe adverse reactions to drugs occur less commonly than in their mild form and that could interfere with defining the standardization of therapeutic measures.
The diagnosis and timely treatment of severe skin toxicities related to checkpoint inhibitor antibodies are essential to optimal multidisciplinary patient therapy, generating a reduction in mortality, as well as long-term sequelae that may occur. It is of utmost importance to identify the causative agent, begin to consider a possible second trigger and potentially differentiate SJS/TEN type reactions from true SJS/TEN to allow patients to continue treatment with ICIs.
None.
The authors declare that there is no conflict of interest.

©2024 Jose, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.