eISSN: 2469-2778


Case Report Volume 11 Issue 4
Oncological surgery service, Spanish Hospital of Mexico City, Mexico
Correspondence: Gallardo Navarro Elias, Oncological surgery service, Spanish Hospital of Mexico City, Mexico
Received: December 02, 2023 | Published: December 18, 2023
Citation: Elias GN, Francisco GR, Carlos MS. Squamous cell carcinoma in situ of the vulva. Hematol Transfus Int. 2023;11(4):119-121. DOI: 10.15406/htij.2023.11.00318
Currently, squamous vulvar cancer is rare, which is why there is limited experience among health personnel in recognizing and managing this type of tumor location. The purpose of this case is to present the case of an 87-year-old woman with an ulcerative lesion on the vulva who underwent left vulvectomy with a report of squamous cell carcinoma in situ pathology, with favorable post-surgical evolution and without the need for adjuvant treatment when the inguinal lymph node was negative for metastasis.
Keywords: vulvar carcinoma in situ, intraepithelial neoplasia, vulvar cancer
Vulvar cancer is a rare neoplasm, it is estimated that it represents 4% of all neoplasms of the genitourinary system. It is estimated that in the United States, according to SEER data, 5,950 women were diagnosed with this neoplasm in 2016 and 1,110 died from it. cause, the incidence is between 65 and 75 years of age and is generally associated with a history of infection by various strains of human papillomavirus, lichen planus, Bowen's disease, among other precursor neoplasms.1 Preinvasive epithelial lesions are characterized by cellular atypia and the possibility of progressing to invasive disease. Currently they are unified within the concept of vulvar intraepithelial neoplasia, their identification and treatment aim at secondary prevention of squamous cancer of the vulva.2 Carcinoma in situ of the vulva was not reported in the Annual Reports until 1985, when 281 cases of stage 0 carcinoma were reported, representing 9.5% of the vulvar neoplasms treated between 1976 and 1978.3 In successive editions, cases of vulvar carcinoma in situ (VIN) range between 10 and 16% of vulvar neoplasms. The most frequent location of the lesions is usually the labia majora, followed by the clitoral region and the vulvoperineal or urethral areas, in 10% of cases it can be multifocal.4 Vulvar cancer in most of its varieties (90%) begins in the skin cells called squamous, the International Society for the Study of Vulvovaginal Diseases (ISSVD) VIN is characterized by the loss of maturation of epithelial cells, associated with hyperchromasia and nuclear pleomorphism, abnormal mitoses and cell crowding. Dyskeratotic cells, hyperkeratosis, parakeratosis and pigmentary incontinence may be present.4,5 The site of metastasis depends mainly on the location, central lesions such as in the region of the clitoris and the perineal and posterior fork have a greater possibility of bilateral inguinal metastases, an aspect that must be taken into account to decide the magnitude of the surgery.
An 87-year-old woman began her current illness after presenting 5 years ago with vulvar excoriation, an erythematous area on the edge of the left labia majora and minora, accompanied by itching, burning, and local pain (Figure 1). She was treated with various topical medications without There is remission of the condition, later an ulcerated lesion without being exfoliative is identified, a reason for consultation. Clinically, the lesion measured 4 x 3 cm in length, located in the region of the left labia minora and majora, reaching the lower third of the vagina and the anoperianal raphe without direct invasion of the vagina or urethra. Macroscopically, the lesion did not protrude from the introitus of the vagina. , no suspicious nodes are palpated in the inguinal regions. No apparent lesions or transcervical bleeding were observed in the cervix, which is why a colposcope was not performed, HPV, HIV negative. General laboratory studies within normal parameters. A biopsy of the area was performed with a report of extensively ulcerated squamous cell carcinoma, so a left hemipelvectomy with sentinel lymph node was performed (Figure 2), with a pathology report of extensively ulcerated squamous cell carcinoma in situ (Figure 3 & 4). the patient presents adequate clinical improvement, the follow-up of the surgical wound is observed for 2 weeks in the office with adequate appearance and healing (Figure 5).
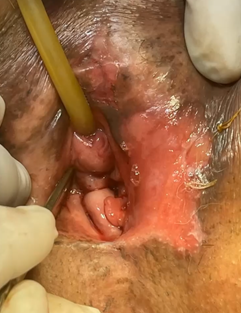
Figure 1 Ulcerated lesion without being exfoliative, reason for consultation. Clinically, the lesion measured 4 x 3 cm in length located in the region of the left labia minora and majora.
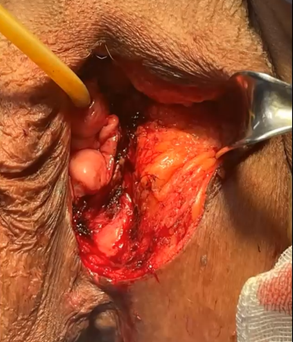
Figure 2 Left hemivulvectomy, surgical resection margin of the entire macroscopic lesion is observed.
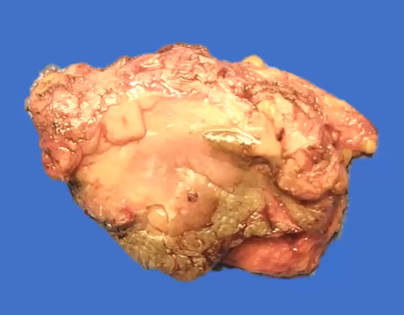
Figure 3 Left hemivulvectomy product: Chronic granulomatous inflammation and body-type giant cells, resection margins: free of neoplasia. peritumoral lymphoplasmacytic infiltrate: yes, mild. areas of invasion, desmoplastic reaction, lymphatic and venous vascular permeation and necrosis: not identified.
The vulva is a place of contact with viral agents such as human papillomavirus (HPV), in addition to being a site of intraepithelial neoplasias such as Bowen's disease or can be affected by extramammary Paget's disease, given the high density of apocrine glands of the anogenital region.5 Its identification and treatment are aimed at secondary prevention of squamous vulvar cancer. Previously, squamous VIN was classified into three grades, similar to cervical intraepithelial neoplasia. Later, it was determined that VIN-1 reflects the generally self-limited infection caused by HPV. In 2004, the ISSVD replaced it with the current single-grade classification system, where high-grade injury is classified as VIN and in the current system VIN is divided into normal or usual type of VIN (includes VIN verrucous, basaloid, and mixed) and differentiated VIN, usual-type VIN is commonly associated with carcinogenic HPV genotypes and other risk factors for HPV persistence, such as smoking and immunosuppressed or immune deficient status, while differentiated VIN is generally not associated with HPV and is more often associated with vulvar dermatological diseases, such as lichen sclerosus, but is more likely to be associated with squamous cell carcinoma of the vulva than the usual or habitual VIN type.6,7 Most cases are identified in the initial stages, as they usually present symptoms such as dyspareunia, pain, bleeding and pruritus; however, significant delays in the diagnosis of this type of cancer have been common due to the lack of some type of screening or the be underdiagnosed with some lesion that is treated with the use of ointments and delays an adequate evaluation.8 Early diagnosis is essential, so any persistent lesion in the vulvar region, whether or not associated with symptoms or a history of HPV infection, must be subjected to a targeted biopsy, which must include epidermis, dermis and connective tissue so that the pathologist can can evaluate the tissue characteristics and depth of the lesion, single lesions are usually not associated with vulvar intraepithelial neoplasia or viruses, although this was not the case in this case, and are usually multifocal when papillomavirus is present.9 No presurgical imaging test has demonstrated effectiveness in diagnosing small lymph node extension. In fact, clinical staging does not detect up to 30% of affected lymph nodes.
The extension can be completed with an abdomino-pelvic tomography in larger tumors. 2 cm, magnetic resonance imaging in locally advanced tumors and a complementary study with extension to the chest or PET-CT when metastasis is suspected. Of the prognostic factors described in squamous vulvar cancer, the validity is lymph node extension and is currently the only one to require adjuvant treatment based on chemotherapy or radiotherapy, the latter consisting of providing radiation to the structures close to the tumor with the objective of reducing the tumor rate. Currently, the standard treatment is based on surgery, always obtaining free margins, however, other less invasive surgical options are taken into account in relation to the degree of tumor invasion and lymph node relationship. In young women, the treatment of this type of lesions is a challenge because The resection should try to affect sexual function as little as possible, which is why surgical ablation with argon or CO2 laser is considered.10 It is estimated that the 5-year survival of squamous cell carcinoma of the vulva is related to the clinical stage is 86.5, 67.7, 40.3 and 21.7% for stages I to IV, respectively.11 Most recurrences occur in the first two years after initial treatment, but up to 40% of vulvar squamous cell carcinomas in early stages recur within 10 years.12 The importance of performing an adequate physical examination of precursor lesions of invasive vulvar cancer, especially lesions that do not resolve and continue to cause symptoms.
Thanks for the images to the clinical pathology service of the Spanish hospital in Mexico City.
The authors declare that there is no conflicts of interest.
None.

©2023 Elias, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.