eISSN: 2469-2778


Review Article Volume 5 Issue 1
1Specialist in Rheumatology, Servicio de Reumatolog
2Specialist in Hematology & Stem Cell Transplantation, Ecatepec Las Am
Correspondence: Jose Eugenio Vazquez Meraz, Specialist in Hematology, Specialist in Stem Cell Transplantation, Regional Director, Ecatepec Las Américas Blood Bank, Av. Carlos Hank González No. 81, Col. El Salado, CP 55055 Ecatepec de Morelos, Edo. De México, México
Received: February 26, 2017 | Published: July 25, 2017
Citation: Duarte-Salazar C, Vázquez Meraz JE. Mesenchymal stem cells: innovative therapeutic intervention for autoimmune rheumatic diseases. Hematol Transfus Int J. 2017;5(1):185-189. DOI: 10.15406/htij.2017.05.00110
Mesenchymal stem cells (MSCs) are multi-potential non-hematopoietic progenitor cells capable of immuno-modulatory activities that diminish adaptive and innate immune responses. MSCs have been the object of multiple publications because their ability to regulate the immunological system and converted MSCs into a promise of developing cell therapies for various autoimmune rheumatic diseases. The present documents summarize clinical studies with efficacy and satisfactory safety profile in which MSCs have been used for cell therapies in systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis.
Keywords: mesenchymal stem cells, cell therapy, systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis
MSCs, mesenchymal stem cells; BM, bone marrow; UC, umbilical cord; AT, adipose tissue; ISCT, international society for cellular therapy; EVs, extracellular vesicles; DC, dendritic cells; SM, soluble molecules; PGE2, prostaglandin E2; TSG-6, TNF stimulated gene-6; NK, natural killer; CAF, cancer associated fibroblast; TNF, tumor necrosis factor; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SS, systemic sclerosis; ANA, antinuclear antibodies; SLEDAI, systemic lupus erythematosus disease activity index
Mesenchymal Stem Cells (MSCs) comprise a subgroup of non-hematopoietic stem cells of the stromal tissue that mediate a wide spectrum of immuno-regulatory activities that usually depress adaptive and innate immune responses. Their ability to regulate the immunological system has converted MSCs into a promise of developing cell therapies for the treatment of various autoimmune diseases. These cells are found virtually in every organ, initially isolated from bone marrow (BM), but can be isolated from umbilical cord (UC), adipose tissue (AT), muscle, synovia, periosteum, cartilage, lymphoid tissue, and dental pulp.1 BM derived MSCs (MSCs-BM) are scarce, constituting <0.01% of the whole cell population of BM, which is the main barrier to their clinical use.2 However, other alternative sites of MSCs are UC and AT, the latter can represent a promising source in which MSCs can be isolated more easily and in greater amounts. According to the definition of the International Society for Cellular Therapy (ISCT), MSCs populations are adherent cells with fibroblast-like morphology isolated from BM that lack of expression of hematopoietic markers such as CD45, CD34, CD14, CD11b, CD79, and CD19 and HLA-DR and expression of CD29, CD105, CD90 and CD73 and exhibit differentiation into osteocytes, adipocytes and chondrocytes.3 MSCs exhibit intermediate levels of molecules class I of the MHC on the cell surface and do not possess detectable levels of class II antigens of the MHC, principally HLA-DR, nor of co-stimulatory molecules CD40, CD80, and CD86. Such low immunogenicity allows MSCs to be an appropriate source of cells for allogenic transplantation,4 explaining why allogenic MSCs may be safely infused to patients without side effects.
Immune regulation mechanisms of MSCs
The mechanisms by which MSCs carry out their immunomodulation function are through cell-to-cell contact, through a structure called MSCs secretome, which includes soluble molecules (SM) and extracellular vesicles (EVs) released by MSCs into the milieu.5,6 The SM mediating immunoregulatory tasks of MSCs are abundant and include indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), soluble HLA-G5 (sHLA-G5), IL-10, TGF- β1, nitric oxide, heme oxigenase-1 (HO-1) and TNF stimulated gene-6 (TSG-6).7,8 These immunoregulatory molecules of MSCs can interact with immunological cells, generating a suppressor response toward T cells, B cells, dendritic cells (DC), macrophages and natural killer (NK).9,10 EVs are subcellular particles of different size comprising exosomes, which originates from multivesicular bodies; these microvesicles are fragments of cell surface membrane and apoptotic bodies. Exosomes are composed of a double layer lipid membrane and inside proteins, lipids and RNAs and, microvesicles are plenty of proteins and lipids.11 EVs can fuse with the surface membrane of adjacent cells and discharge their content, promoting or regulating functional changes in target cells.12 Exosomes can participate in various diseases notably remodeling the tumor environment by cancer associated fibroblast (CAF) generation, neo-angiogenesis, tumor proliferation and metastasis.13
MSCs are capable of regulating both adaptive and innate immunity through these immuno-regulatory molecules. The principal immuno-regulatory effects of MSCs are exerted in adaptive immunity to T cells as follows:
MSCs also act on B cells inhibiting their proliferation, differentiation and activation, with a limited production of immunoglobulins. This effect is also mediated by T cells19,20 (Figure 1). On the other hand, in the innate immunity system, there is the participation of mature DC (mDC), which plays a primordial role in the presentation of naive T-cell antigens. It has been demonstrated that mesenchymal cells interfere with DC function in the presentation of antigens through diminishing the expression of class II molecules and co-stimulatory molecules, as well as the decrease of the production of IL-12, whose main action is that of promoting the differentiation of naive helper T cells into the Th1 subgroup for IFN gamma production.21,22 Additionally, MSCs down-regulate the proinflammatory potential of DC on inhibiting tumor necrosis factor (TNF) production.17 On the other hand, plasmacytoid DC (pDC), a rare DC subtype, are specialized cells that express Toll-like receptors 7 and 9, which permit the detection of viral and bacterial nucleic acids. These cells produce great amounts of type I IFN in response to the microbial stimulus; however, the production of IL-10 increased when these cells are incubated with MSCs.23 MSCs suppress the effector functions of neutrophils and NK cells and, modify monocyte differentiation toward an anti-inflammatory phenotype, inducing macrophage 2 (M2).24 This interaction between MSCs and immune system, both adaptive and innate system, generates regulatory effects on immune cells, rendering MSCs as an interesting and potential therapy in autoimmune disorders (Figure 1). The purpose of this review is to present clinical studies with clinical efficacy and satisfactory safety profile of MSCs in autoimmune rheumatic diseases such as Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA) and Systemic Sclerosis (SS).
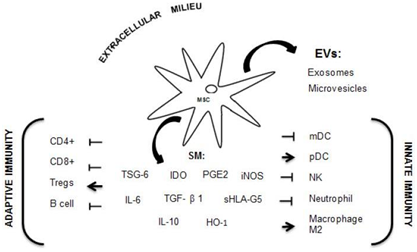
Figure 1 Mechanism of immunomodulation through MSCs releasing soluble molecules (SM) and EVs (extracellular vesicles) interacting with immunological cells from adaptive and innate systems.
Systemic lupus erythematosus
Current therapies are rarely curative in patients with severe forms of this autoimmune disease. In SLE, conventional treatment with corticosteroids and immunosuppressors or with biological agents, have improved the outcome in these patients.25 However, there is a subgroup of patients with important mortality and morbidity who do not respond to medical treatment, reporting a mortality of up to 10% at 10years.26 Scientific evidence revealed that MSCs-BM in animal models as well as in patients with SLE present deterioration in their capacity of proliferation, differentiation and secretion of cytokines, as well as their immunomodulatory mechanisms. The MSCs-BM of patients with SLE exhibit early signs of senescence, observing flat and larger mesenchymal cells with slow growth in cell cultures as compared with that of control MSCs.27 They also show a reduced ability for producing T regs cells28 and an increase in the proportion of MSCs apoptosis.29
From 2010 to the present, have been reported in the literature 280 SLE patients refractory to conventional treatment and treated with MSCs30 predominantly with severe lupus nephritis. Consequently, SLE was attenuated reducing clinical manifestations, stabilization of renal damage and improvement in serological markers (Table 1). Initially, autologous MSCs-BM efficacy and safety were evaluated in two patients with SLE, observing an important increase in T regs cells, without improvement in clinical manifestations.31 Another report, published 15 patients with SLE with predominance of renal activity; the patients received an intravenous infusion of 1×106cells/kg of allogenic BM-derived MSCs. At 12months of follow-up, Systemic Lupus Erythematosus Activity Index (SLEDAI) scores diminished from 12.2±3.3 to 3.2±2.8, proteinuria from 2,505±1,323 to 858±800mg/24h, and a reduction in anti-double-stranded DNA (dsDNA) levels.32 In 16patients, allogenic UC-derived MSCs were infused in patients with lupus nephritis; these patients were administered 1×106 cells/kg. SLEDAI scores diminished significantly one month after infusion with MSCs-UC, 18.4±1.1 vs. 10.8±0.8, the scores continuing to improve after the third month. Proteinuria, anti-dsDNA antibodies, and antinuclear antibodies (ANA) diminished from the third month on, while T regs cells increased.33 Alveolar pulmonary hemorrhage and hematological abnormalities, that compromise life were treated with MSCs-UC and MSC-BM respectively. All patients presented improvement at one month.34,35 From the same research group, Wang D et al.36 included 87patients with refractory SLE with a risk of organ failure; these patients received an infusion of MSCs-BM of allogenic UC at a dose of 1×106cells/kg. The authors reported 28% of patients in complete remission at 1year; 31% at 2years, 42% at 3years and 50% at 4years. Frequency of relapse was 12% at 1year, 18% a 2years and 17% at 3 and 4years36 and Gu et al.37 utilized the MSCs BM of relatives in 81 patients with lupus neprhirtis, reporting complete remission in 60% of patients, with an important decreased in the SLEDAI score, an increase in glomerular filtration, and reduction of the dose of prednisone, cyclophosphamide, and mycophenolate mofetil.37 More recently, in a study to evaluate the safety and efficacy of MSCs-UC in patients with active SLE, the patients received an infusion of 1×106cells/kg on days 1 and 7. The results were the following: 60% with complete or partial response during a 1-year follow-up. Seven patients presented relapse after 6 months. The baseline SLEDAI score was 10.83±4.63, while the final SLEDAI score was 6.4±3.5, with diminution of anti-dsDNA and ANA with normalization of C3.38 Thus, these data indicate that treatment with allogenic BM or UC-derived MSCs in a subgroup of patients with SLE who do not respond to conventional treatment demonstrated significant reduction in disease activity and improvement in immunological parameters. In comparison the first study reported on two patients with SLE in which autologous MCSs were utilized did not demonstrate clinical benefits.31 This initial information strongly suggests that transplantation with allogenic MSCs of BM and/or UC nor autologous can provide an important benefit in patients with this autoimmune disease.
Author |
No. of |
Clinical |
Origin of |
Outcome |
Follow Up |
Carrion F et al. 31 |
2 |
Heterogeneous |
Autologous |
Increased T regs |
14 |
Liang J et al.32 |
15 |
Nefritis |
Allogenic |
Decreased |
12 |
Sun L et al.33 |
16 |
Nefritis |
Allogenic |
Decreased |
8 |
Shi D et al.34 |
4 |
Alveolar |
Allogenic |
Increased |
6 |
Li X et al.35 |
35 |
Cytopenias |
Allogenic |
Increased |
21 |
Wang D et al.36 |
87 |
Heterogeneous |
Allogenic |
Clinical |
27 |
Gu F et al.37 |
81 |
Nefritis |
Allogenic |
Renal remission 60% |
12 |
Wang D et al.38 |
40 |
Nefritis |
UC-allogenic |
Complete clinical |
12 |
Table 1 Outcome of 280 SLE patients treated with Mesenchymal Stem Cells (MSCs)
Rheumatoid arthritis (RA)
RA is a chronic autoimmune inflammatory joint disease characterized by synovial hyperplasia and joint damage leading significant disability. Current treatment strategies do not arrest cartilage and bone damage in spite of clinical remission. MSCs have immunomodulatory and tissue repair properties and, their use in RA is being explored. In RA allogenic MSCs were used with clinical improvement but no more than few months suggesting that cell therapy in RA would require repeated infusions. Since 2012, almost 200 patients with refractory RA were treated with MSCs.39–41 All treated patients had clinical improvements with no serious adverse effects reported (Table 2).
Author |
No. of |
Clinical |
Origin |
Outcome |
Follow Up |
Other conditions |
Liang J et |
4 |
Refractory |
Allogenic BM |
Reduced DAS 28 and |
19 |
No immunological |
Wang L |
136 |
Refractory |
Allogenic UC |
Improved DAS 28 and HAQ |
8 |
Repeated |
García Álvaro |
46 |
Refractory |
Allogenic AT |
Approximately 30% of each group |
6 |
Repeated infusions |
Table 2 Outcome of 186 RA patients treated with Mesenchymal Stem Cells (MSCs)
Sclerosis systemic (SS)
SS is a disease characterized by early vascular endothelial damage, consequently with an increase in collagen synthesis and progressive fibrosis of the skin and internal organs. This autoimmune disease exhibits a mortality ranging between 30 and 50% during the first 5years.42 At present, there is no treatment that is useful in SS, except for autologous hematopoietic cell transplantation, which evidences greater therapeutic benefit and an increase in survival in comparison with treatment with monthly dosages of cyclophosphamide.43 Considering the pleiotropic effects of MSCs with immunoregulation, angiogenic and anti-fibrotic capacities, these cells can represent an opportunity in this intractable disease. Scientific data demonstrates that MSCs-BM in patients with SS present deterioration in their immunomodulatory mechanisms. The MSCs-BM of patients with SS demonstrates premature senescence; however, they retain suppressor functions and a normal ability to generate functional T regs cells.44 Recently, it was demonstrated a significant increase of transforming growth factor (TGF)-β receptor type II in the mesenchymal cells of patients with SS compared with mesenchymal cells of healthy donors. This increase associated with an activation of TGF-β, generates an increase in the synthesis of the gene that encodes type I collagen, thus, an excessive production of type I collagen is unfavorable in this disease.45 These findings show that the mesenchymal cells of patients with SS are defective in certain functions; therefore, the use of the allogenic MCSs of healthy individuals is justified in these patients.
In 2008, Christopeit et al.46 reported important improvement in a female patient with refractory SS at post-transplantation of MSCs-BM of a related haploidentical allogenic donor. The infusion of MSCs was associated during the following 3 months with cicatrization of the digital ulcers at six months with improved blood flow in the fingers and an increase of partial transcutaneous oxygen pressure; as well as improvement in the score of skin measurement according to the Rodnan scale (11 vs. 25points), but without changes in immunological parameters.46 In 2011, this same study group reported five additional cases of patients with this disease treated with allogenic MSCs-BM. These patients exhibited fibrosis of the skin (5/5), interstitial pulmonary disease (4/5), vasculopathy (5/5), cardiomyopathy (4/5), and myopathy (2/5). At 18months of follow-up, 4/5 of the patients improved in skin thickness, in digital ulcers, or in limb necrosis.47 Limb revascularization was reported in a patient with SS after autologous MSCs-BM infusion.48 More recently, in a phase I study, the safety of autologous MSCs-AT was demonstrated in patients with severe lower-limb ischemia without the possibility of revascularization, exhibiting an increase in transcutaneous oxygen pressure and leg ulcer cicatrization.49 Local injections of autologous MSCs of peripheral blood and BM have been utilized, reporting an improvement in the Raynaud phenomenon and of digital-ulcer and lower-limb cicatrization.50 Autologous MSCs-AT have been employed in local injection of areas localized of the face, with a reduction of skin thickness.51 and in that of the fingers, with important improvement of the disability, of the edema and of the Raynaud phenomenon.52
Despite that knowledge remains limited, clinical studies suggest that these MSCs, due to their immunomodulatory properties, can be relevant in the treatment of autoimmune clinical conditions that compromise life, such as SLE, RA and RA. Additionally, MSCs presents an adequate safety profile in terms of their administration, because adverse serious events attributed to their administration have not been reported in these diseases. The immunoregulation capacity of MSCs confers to these cells a very promising therapy in these autoimmune rheumatic diseases. These data justify considering MSCs as an innovative therapy. Finally, it is indispensable for long term studies to be performed to determine whether these immunomodulators effects of MSCs are transient or long-lasting.
None.
The author declares no conflict of interest.

©2017 Duarte-Salazar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.