eISSN: 2469-2778


Case Report Volume 9 Issue 2
1Department of Neurology, Instituto Mexicano de Neurociencias, Hospital Ángeles Lomas. Huixquilucan, Estado de México, México
2Department of Neurosurgery, Instituto Mexicano de Neurociencias, Hospital Ángeles Lomas, Huixquilucan, Estado de México, México
3Department of Hematology, Hospital Ángeles Lomas. Huixquilucan, Estado de México, México
Correspondence: Jacobo Lester, MD, Vialidad de la Barranca #22, Consultorio 750, Col. Valle de las Palmas C.P. 52787, Huixquilucan, Estado de México, México
Received: March 29, 2021 | Published: April 21, 2021
Citation: Lester J, Klériga E, Pérez-Zincer F. Low dose of Dasatinib in chronic myeloid leukemia. Hematol Transfus Int J. 2021;9(2):35-36. DOI: 10.15406/htij.2021.09.00249
The oncoprotein BCR-ABL1 helps with the diagnosis and monitoring the therapeutic response in patients with chronic myeloid leukemia. We describe the case of a 70-year-old woman with chronic myeloid leukemia who did not achieve a molecular response after 12 months of treatment. She was then managed with Dasatinib 70 mg orally twice a day until she reached a complete molecular response. She developed pulmonary toxicity probably due to Dasatinib. The dosage was decreased to 70 mg orally once a day. The patient continues with a complete molecular response with less side effects.
In chronic myeloid leukemia, as far as we know, there are no previous reports, after a molecular response with Dasatinib 70 mg twice a day, the maintenance of a good molecular control with a single daily dose. This dose may allow a reduction in toxicity with a better quality of life for patients with chronic myeloid leukemia.
The fusion of the Abelson murine leukemia gene (ABL1) on chromosome 9 with the breakpoint cluster region (BCR) gene on chromosome 22, known as the Philadelphia chromosome, has been postulated as the etiology of the chronic myeloid leukemia (CML). This translocation results in the expression of the oncoprotein called BCR-ABL1, causing leukemogenesis with a cell cycle and aberrant cytosine production.1 The recognition and measurement of this oncoprotein helps with the diagnosis and monitoring -the therapeutic response in patients with this disease.2
This is the case of a 69-year-old woman with a history of Gold IV chronic obstructive pulmonary disease secondary to heavy chronic smoking (120 packs per year for 40 years, stopped in 2010), Sjögren's syndrome diagnosed in 2013, seizures due to levofloxacin in 2014 and, chronic myeloid leukemia (CML) diagnosed in February 2017 studying leukocytosis in “check-up”. In March 2017, she started oral treatment with Imatinib 400 mg once a day with side effects such as: excessive fatigue, nausea and frequent vomiting, without being able to achieve molecular response (BCR / ABL1) 12 months after starting treatment. This was replaced by Dasatinib 70 mg orally twice a day in March 2018. She presented better tolerance and reached a complete molecular response in October 2018. On January 14, 2019, the patient was hospitalized for pneumonia, multiple foci, with poor initial response to antibiotics. She maintained complete molecular response (BCR-ABL1 of 0.0%) during this time. In addition to pneumonia, pulmonary toxicity due to Dasatinib was suspected and the Dasatinib dose was decreased to 70 mg orally once a day. This Dasatinib dosage has been continued for 28 months after the CML entered in remission. The patient continues with a complete molecular response (BCR-ABL1 of 0.0%).
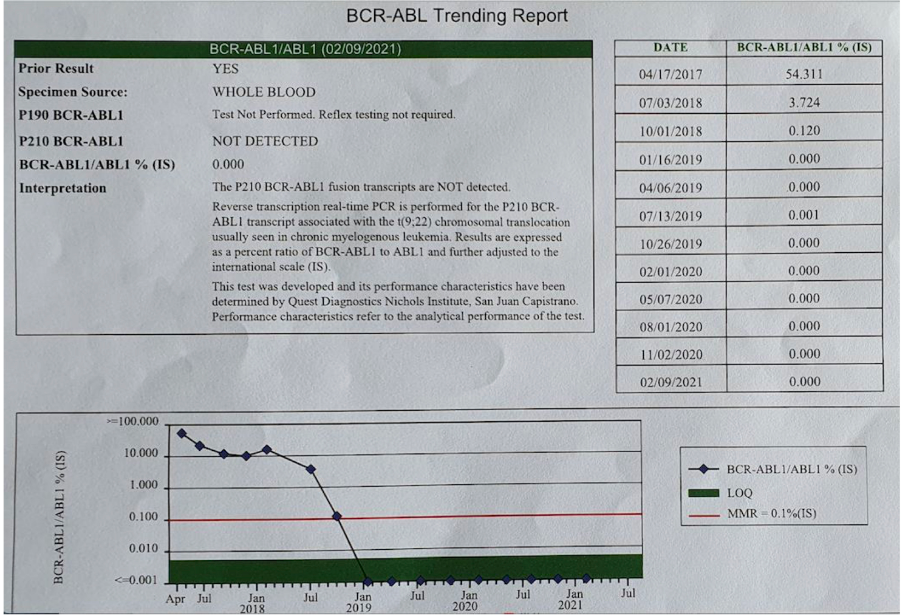
Figure 1 Report over time of BCR-ABL1.
Date of diagnosis: February 2017
Imatinib 400 mg once daily: March 2017 to March 2018
Dasatinib 70 mg twice a day: March 2018 to January 2019
Dasatinib 70 mg once daily: January 2019 to present
Submission deadline: October 2018
Full remission: January 2019
Last measurement: February 2021.
It is known that 50% of patients with CML are asymptomatic and are diagnosed by blood tests performed for some other reason,2 as has been the case in our patient. CML treatment - with tyrosine kinase inhibitors (TKI), interferes with the interaction of the BCR-ABL1 oncoprotein and adenosine triphosphate (ATP), blocking the cell proliferation of the malignant clone. The 10-year survival of the disease is about 80–90%.2 Imatinib was the first TKI approved by the FDA3 and it is considered the treatment of choice with the usual dose of 400 mg once a day with a 10-year survival of 83.3%.4
Second-generation TKIs are more powerful but also have more adverse effects and toxicity than Imatinib.5 Dasatinib and Nilotinib are the second drugs generation indicated for failure or intolerance to Imatinib. Dasatinib dose is 70 mg orally twice a day,2 however, there are reports that doses of 100 mg once a day can induce a quick and profound response with a molecular response at 3 months. The response rate is 64% for Imatinib and 84% for Dasatinib, with a 5-year overall survival practically the same, 91% and 90% respectively.6
The adverse effects of Dasatinib are apparently proportional to the dose,7 such as pleural effusion in 19%, myelosuppression in 20% and pulmonary hypertension in 2%.8
A 70 mg orally dose twice a day and 100 mg once a day results in a similar response.8 When there is intolerance to Imatinib, Dasatinib should be considered. Comparative studies have been carried out with Dasatinib at different doses, 100 mg once a day, 50 mg twice a day, 140 mg once a day and 70 mg twice a day, with a 5-year total survival rate of 71 -74-77-70% and progression-free survival of 49-51-40-47% with each dosage at 6 years of follow-up, respectively.9
Naqvi K et al.10 reported that Dasatinib, 50 mg once a day in patients with newly diagnosed CML, 93% achieved an early molecular response (MMR) at 3 months of follow-up with PCR for BCR-ABL1 <10%. Fluorescent in situ hybridization (FISH) showed that for 70% of patients the presence of this translocation was negative at 3 months; the remaining 30% did not make it negative for 6 months.10
Based on our experience, we propose the possibility of using Dasatinib 70 mg twice a day until a complete molecular response is achieved and then reducing the dose to 70 mg once a day to reduce the adverse effects of the drug.
To our knowledge, there are no previous reports that after achieving a complete molecular response in CML with Dasatinib, we may continue with a maintenance dose of 70 mg orally every 24 hours. after reaching the complete molecular response with the standard dose of 70 mg orally twice a day. We propose to explore Dasatinib 70 mg orally as a long-term maintenance dose when there is toxicity at standard doses. This may allow a reduction in toxicity with a better quality of life for patients with CML. It is our believe that more studies should be done to confirm our findings.
The authors declare that there are no conflicts of interest relevant to this work.
None.

©2021 Lester, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.