eISSN: 2469-2778


Case Report Volume 9 Issue 5
Department of Pediatric Hematology, Faculty of medicine, Suez Canal University, Egypt
Correspondence: Samar Mohamad Elfiky, MD, Lecturer of Pediatrics, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
Received: August 09, 2021 | Published: September 29, 2021
Citation: Elfiky SM, Sahmoud SI. Dubin Johnson Syndrome associated with defective ristocetin – induced platelet agglutination. Hematol Transfus Int J. 2021;9(5):90-92. DOI: 10.15406/htij.2021.09.00262
Background: This is the first reported case of platelet aggregation defect in association with Dubin-Johnson Syndrome.
Case presentation: We reported a 14 months old female infant with recurrent attacks of mild to moderate ear and nose bleeding and moderate amount of subarachnoid hemorrhage with ristocetin induced platelet agglutination other than Von Willebrand Factor disease and Bernard–Soulier syndrome. The patient also had fluctuating bilirubin level (Maximum of 15 mg/dl) and remaining liver function tests were normal; the patient diagnosed as Dubin Johnson Syndrome by liver biopsy.
Conclusions: Dubin-Johnson Syndrome may be associated with platelet aggregation defect.
Keywords: Dubin-Johnson Syndrome, platelet aggregation defect
DJS, dubin johnson syndrome, ITP, immune thrombocytopenic purpura
Dubin—Johnson syndrome (DJS); is an autosomal recessive disorder characterized by chronic mild conjugated hyperbilirubinemia, without other features of hepatobiliary disease (OMIM*237500).1 It was found that DJS results from mutations in the protein encoded by the gene ABCC2 that helps in the transport of organic anions into the bile canaliculi.2,3 The decreased canalicular and increased intracellular ABCC2; decreases the bile secretion thus cause cholestasis.4 DJS was reported in association with some causes of bleeding tendency as factor VII deficiency,5,6 immune thrombocytopenic purpura (ITP).7 Other case reports found an association between DJS and systemic lupus erythematosus, and hepatic cavernous hemangioma.8,9 To the best of our knowledge, no DJS cases were reported in association with platelet aggregation defect.
Our 8 month female patient was the 5th in order for a positive consanguineous parents (cousins), her pregnancy passed uneventful and delivered by normal vaginal delivery at term without complication, the immediate postnatal period also was free from any complications or drug intake. At the age of 15 days, she developed multiple ecchymotic patches all over the body, pallor, and depressed activity. At this time initial bleeding screening revealed normal platelet count 250.000, PT, and PTT. However, her hemoglobin (Hb) was 10gm % and CT brain showed moderate subarachnoid hemorrhage. Despite of the normal coagulation profile she received intramuscular vitamin K and fresh frozen plasma transfusion and 4 days later, she regained her full activity and was discharged.
At the age of 45 days, she presented to our hematology clinic with history of 4 to 5 attacks of epistaxis and external ear bleeding since NICU discharge. Complete blood count (CBC) (HB =11 gm /dl, Plt= 285000 c/cmm, TLC= 10.500c/cmm); PT = 13 sec (control is 13), APTT= 35 sec (control is 37 sec) were normal; but bleeding time was more than 10 minutes. Accordingly Von Willberand Factor (VWF) antigen and ristocetin cofactor activity and platelet aggregation tests were done. Von Willberand Factor antigen and activity were normal (160%, and 113 %), platelet function tests showed decreased aggregation response to ristocetin 33% (normal= 70- 120%) with slight reduction on aggregation response to collagen 60% (normal= 70- 120%), and normal aggregation response to ADP 105% (normal= 70- 120%). Bernard-Soulier syndrome was suspected but glycoprotein (Gp) Ib/IX/V was normally expressed. Bleeding transiently stopped after platelet transfusion and the infant was discharged for follow up.
At the age of 5 months, she was still having recurrent mild to mod attacks of bleeding that stops with platelet transfusion. However she presented with new complain of jaundice and clay stool. Clinical examination at that time revealed mild jaundice, no pallor, no ecchymotic patches, no hepatomegaly or splenomegaly. CBC values were as follows: HB= 9 gm% , TLC =7000c/cmm, PLT= 400.000 c/cmm; total bilirubin was 9.4 mg/dl with 8.1 mg/dl direct bilirubin; liver enzymes were normal ; ALT = 24 U/L, AST= 34 U/L, GGT= 28 U/L (normal= (5- 85), Alkaline phosphatase= 316 U/L normal (4-140 ), serum albumin = 3.8 gm/L, PT = 11 sec, PTT = 30 sec, serology for hepatitis A, B and C antibodies were negative, and TORCH screen showed positive Toxoplasma IgG, while rubella and cytomegalovirus immunoglobulin were negative. Abdominal ultrasonography was also normal. However, one week later serum bilirubin levels declined to 3.2 mg /dl total and 2.7 mg/dl direct bilirubin and stabilized at almost the same values for almost two months before direct bilirubin surged to 13.8 gm/dl with normal liver function. Abdominal ultrasonography had been repeated and again showed normal liver size and texture with no signs of intrahepatice biliary atresia so Tc99m IDA hepato-biliary scintigraphy was done and it ruled out extrahepatic biliary atresia with only delayed hepatic to bowel tracer transit (Figure 1). Urinary Coproporphyrins were not available, and liver biopsy was planned.
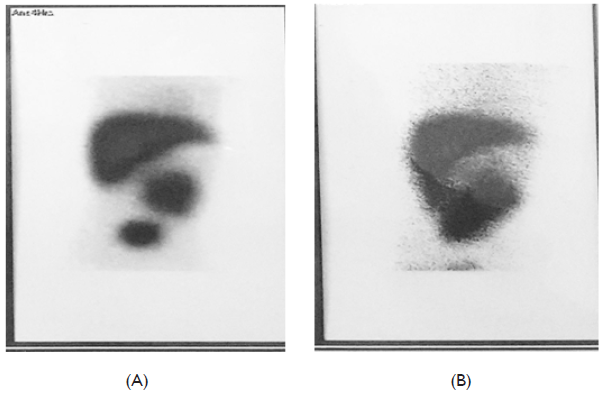
Figure 1 Tc99m IDA Hepato-Biliary Scintigraphy.
(A) The tracer appeared in the bowel in 5 hours image. (B) the tracer filled the bowel lobes in the 24 hours image.
Before liver biopsy platelet functions tests was repeated revealing normal platelet aggregation in response to ADP (81%) and a much-reduced response to ristocetin (10%). Liver biopsy procedure passed with no bleeding as the infant received platelet transfusion before. The histopathology examination depict that liver cells were free from any necrotic or inflammatory lesions but there was microvascular steatosis and coarse brown black pigment in the perivenular liver cells with Kupffer cells hyperplasia suggesting the diagnosis of Dubin-Johnson Syndrome (Figure 2).7–9
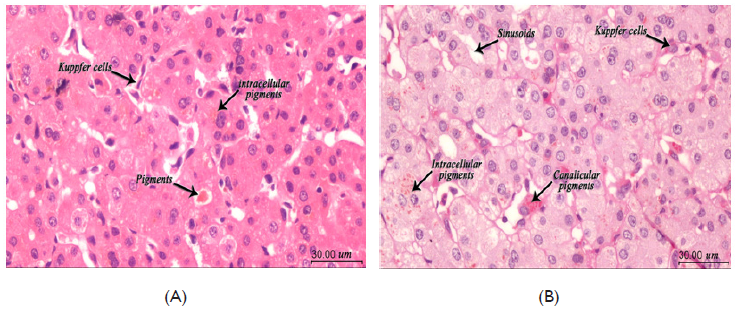
Figure 2 Liver biopsy.
(A) H&E examination at 400x magnification. Liver tissue with preserved architecture, hyperplasia of Kuppfer cells, accumulation of pigment inclusions both intracellular and cancalicular. (B) PAS stains at 400x magnification. Highlights the pigments even with few foci. All Images captured using: Calibrated standard digital microscope camera (ToupTek® XCAM1080PHA digital microscope camera) using Olympus® CX21 microscope, with resolution of 5 MP (megapixels) 1920 x 1080 pixel. "ToupView" software for capture and image enhancements.
The physiological role of platelets is to help hemostasis at sites of vascular injury through formation of platelet plugs that adhere to the exposed subendothelial collagen and to collagen-immobilized von Willebrand factor (VWF). Platelet adhesion initiates activation events that spread to increase surface contact.10 The prevalence of inherited platelet disorders is unknown. A survey of pediatric centers in Germany, Austria, and Switzerland estimated two affected children per million populations.11 Mild platelet function disorders (PFDs) are complex and difficult to diagnose, most affected individuals have mucocutaneous bleeding including bruising, epistaxis, bleeding from oropharynx or gastrointestinal tract.12 Bernard–Soulier syndrome (BBS) BBS is an autosomal recessive disorder (OMIM*231200), its diagnostic criteria of BSS are giant platelets, defective ristocetin-induced platelet agglutination, a lack of VWF dependent platelet adhesion to subendothelium.13 Platelet counts in BSS mostly range from 40 000 /L to near normal.14 BBS is the product of four distinct genes (Iba, Ibb, IX andV). The genes for GPIba and GPIbb are on chromosomes 17 and 22, while those encoding GPIX and GPV are on chromosome 3. The GPIba gene is most frequently affected in BSS.15 In our case we found a defective ristocetin-induced platelet agglutination but with normal platelet morphology and count beside a normal GP 1b- IX complex. We faced the limitation of the inability to do gene mapping for BBS. Noticeably all the bleeding attacks in our infant was completely controlled by platelet transfusion. The fore-mentioned work up of the direct hyperbilirubinemia in our infant reached the diagnosis of Dubin Johnson syndrome and we found no previous reported cases of the same association thus a concurrent association is suggested.
This is the first reported familial case of DJS with platelet aggregation defect.
We would like to thank our colleagues from the biochemistry, immunology, histopathology laboratory departments as well as the intervention radiology arm in taking the liver biopsy.
The authors declare no conflicts of interest.

©2021 Elfiky, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.