eISSN: 2469-2778


Case Report Volume 11 Issue 4
1Oncological surgery service, Mexico
2General surgery, Spanish Hospital of Mexico City, Mexico
Correspondence: Gallardo Navarro Elias, Oncological surgery service, Spanish Hospital of Mexico City, Mexico
Received: December 03, 2023 | Published: December 19, 2023
Citation: Elias GN, Carlos MS, Rodriguez FG. Clear cell carcinoma of Müllerian origin. Hematol Transfus Int. 2023;11(4):123-125. DOI: 10.15406/htij.2023.11.00319
Clear cell carcinoma is structures of Mullerian origin, they are associated with mutations in TP53 as they are a high-grade neoplasm, however there is no immunophenotypic pattern that guides us if the origin is from the ovary, uterus, endocervix or endometrium. A 71-year-old female patient who underwent surgery for a right parauterine tumor with a pathological report that corresponded to a clear cell tumor. Subsequently, a staging and cytoreductive laparotomy was performed, performing abdominal cavity lavage, salpingo-oophorectomy of the remaining ovary (left), and pelvic and paraortic lymphadenectomy. , omentectomy and biopsies of the parietal peritoneum, all the material was negative for metastatic disease, 18 months later there was a relapse in the vaginal vault, with the presence of an ovoid tumor measuring 10 cm in diameter, treated with colpectomy, with a report of clear cell carcinoma with CK7+ / Napsin A+ / p53+ protein immunophenotype, with a satisfactory disease-free postoperative period.
Keywords: clear cell carcinoma, mullerian epithelium, immunohistochemistry
The pathogenesis and origin of clear cell müllerian carcinomas currently remain largely a mystery, clear cell carcinoma (CCC) is observed in tissues originating from the paramesonephric ducts such as the kidneys, ovaries, cervix and the vagina, therefore its location varies in relation to the structures derived from these.1 In the ovary they represent less than 5%, being associated with endometriotic cysts in up to 24%, establishing a relationship between endometriosis and clear cell carcinoma 6 times greater than that found between endometriosis and other ovarian carcinomas,2 on the other hand. The endometrium is divided into two broad categories, type I or endometrioid carcinomas, which are associated with high levels of estrogen, and type II, which includes clear cell carcinoma, which is generally not related to hormonal events. Firstly, it is associated with mutations of PTEN, KRAS and PIK3CA and type II carcinomas are associated with mutations in TP53. In vaginal cancer, clear cell histology represents less than 10%, and endometrioid, mucinous and mesonephric adenocarcinomas can also be found,3 in the cervix, clear cell adenocarcinoma is a disease that represents only 2 to 7% of all cervical adenocarcinomas,4 the factors associated with the development of this neoplasia are women exposed to diethylethylbestrol and in women not exposed, the factors are microsomal instability, human papillomavirus infection, overexpression of Bcl-2 and mutation of the p53 gene.5 Homologous tumors are those neoplasms whose stromal component derives from the Müllerian tissue and heterologous tumors are those that present a stromal component differentiated from cell types not associated with the Müllerian duct system, such as striated muscle, bone and cartilage.6 It seems that Mullerian CCCs may have variable pathogenesis depending on their nuclear grade and association with some pathologies such as endometriosis. These tumors develop a clinical course and aggressive biological behavior than the rest of the histological subtypes such as ovarian tumors, regardless of their degree of differentiation.7 Histologically, the cells are clear, with abundant cytoplasm loaded with glycogen and cells with a horseshoe nail characteristic, with little cytoplasm, prominent nuclei, which are arranged in a solid, tubulocystic, papillary pattern, or in a combination of these.8 The histopathological features of importance in determining the prognosis of clear cell adenocarcinoma are stage, tumor size, growth pattern, nuclear atypia, and mitotic activity.
A 71-year-old woman, with a history of type 2 diabetes mellitus, high blood pressure, who underwent surgery in another hospital due to noticing an abdominal tumor mass predominantly on the right, underwent total abdominal hysterectomy with right salpingo-oophorectomy preserving the left ovary, the report the pathology corresponded to a clear cell tumor. Subsequently, a review of the slides was performed in our hospital, which confirmed the diagnosis. However, the origin was not located if it was from the endometrium, uterus and ovary. Origin was assumed to be Mullerian remains. A staging and cytoreductive laparotomy was subsequently performed at our institution, performing lavage of the abdominal cavity, salpingo-oophorectomy of the remaining ovary (left), pelvic and paraortic lymphadenectomy, omentectomy and biopsies of the parietal peritoneum. All material was negative for metastatic disease. Adjuvant chemotherapy was considered, administering 6 cycles of carboplatin and pacitaxel with adequate tolerance. She was kept under observation, for 18 months she had urinary incontinence and a sensation of a foreign body in the vaginal area. During the physical examination, an increase in volume was palpable in the suprapubic region, so an abdominal computed tomography was requested, which showed a 10 cm ovoid tumor (Figure 1) in diameter in the center of the pelvis behind the bladder and in front of the rectum, tumor recurrence was considered. No para-aortic or iliac lymph nodes were seen. Complementary preoperative studies were performed without any anatomicalities. In particular, the gynecological examination was normal. The patient underwent midline laparotomy, with resection of the tumor and definitive study, with a report of clear cell carcinoma, positive cytokeratin 7, positive napsin A, positive p53 protein (Figure 2 & 3). The patient was discharged in adequate condition 3 days later, free of illness.
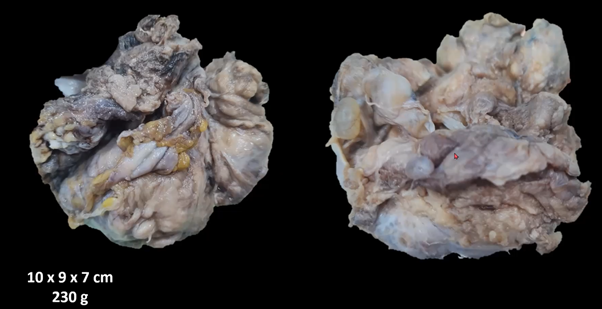
Figure 1 Ovoid tumor of 10 cm diameter in the center of the pelvis behind the bladder and in front of the rectum clear cell carcinoma, Immunophenotype: CK7+ / NAPSIN A+ / PROTEIN p53+.
The majority of clear cell adenocarcinomas of the cervix are of Müllerian origin; these can involve any portion of the vagina, cervix, or both, although approximately 60% of these lesions are confined to the anterior wall of the upper third of the vagina,9,10 when these cells occur in the subtypes of ovarian carcinoma, the differential diagnosis can be established by morphological characteristics and H&E staining, because they may have some peculiarities that overlap morphologically, especially high-grade serous adenocarcinoma, clear cell and endometrioid.11 The positive diagnosis of this entity is based on the microscopic and immunohistochemical study of the sample; some authors require the presence of at least 50% of the clear cell contingent to consider the diagnosis of CCC.12 One of the immunohistochemistry markers is PAX-8 (Paired box protein 8), a member of the family of transcription factors that regulate organogenesis and maintain the function of the thyroid gland, kidney, central nervous system (eyes, middle ear ), Müllerian and mesonephric ducts,13,14 consistently have nuclear expression of the marker of Müllerian origin WT1, similar to the epithelium of the uterine tuba, also the CCC, which are similar to endometrioids, are positive for HNF-1β and napsin-A, and with high frequency negative for estrogen and progesterone receptors, napsin-A is positive by immunostaining in the cytoplasm of neoplastic cells and WT-1 is negative in neoplastic cells.15 In 90% of cases, CCCs with p53 mutations have been reported that show a native staining pattern, that is, weak and focal of the nucleus of neoplastic cells, estrogen and progesterone receptors usually show positivity for low-grade endometrioid carcinoma or intermediate grade, and negativity for clear cell carcinoma.11,16 Unlike adenocarcinomas in the endocervix and endometrium, we can sometimes rely on immunohistochemistry for the expression of the vimentin protein, since if they express vimentin it is most likely from the endometrium and if they do not express it it is probably from the endocervix because They have very high expression of the Tp53 protein, in clear cell carcinoma there is no marker that can tell us if the origin is from the endometrium, endocervix or ovary, since there is no specific marker, sometimes, as in our case, the origin is considered of the Müllerian epithelium, the expression of the Tp53 protein in this tumor is only because it is a high-grade carcinoma, so the Tp53 protein in this case its expression only corroborates the malignancy, some way to sometimes differentiate it is through immunoreactivity.
The nuclear pattern of these neoplasms is characteristic of serous ovarian carcinoma, both high and low grade, unlike endometrioid and clear cell carcinomas because they are negative (10). Most endometrial CCCs also express cytokeratin 7 (CK7). CAM 5.2, 34βE12, carcinoembryonic antigen (CEA), Leu-M1, vimentin and CA-125. Endometrial clear cell carcinoma is very aggressive with a five-year survival rate of 30% to 75%.17 Macroscopically, these tumors appear solid, nodular or lobulated masses. Most tumors have a soft and shiny capsule pale yellow or a light gray external surface.17 Then, immunohistochemistry in neoplasms of the female genital tract is performed based on a comprehensive diagnosis, guided by clinical data and morphological pattern. It is important to recognize that immunohistochemical markers have prognostic and predictive implications, but they are not a determining factor in the patient's treatment. The staging procedure in this type of neoplasms is laparotomy, which should include collection of ascites or washing cytology for staging, thorough examination of all peritoneal surfaces, hysterectomy, bilateral salpingo-oophorectomy, omentectomy, lymph node sampling, and peritoneal biopsies, as in our case,10 then radical surgery appears as the most successful initial treatment, followed by adjuvant chemotherapy or radiotherapy or combined chemoradiotherapy that could be an effective treatment.6,16 The prognosis is unfavorable if it is related to increased tumor activity, nuclear atypia and high mitotic activity, so it is necessary to define each treatment, and correlate it with this molecular tool and morphometric characteristics, to determine more precisely and earlier, the real risk of these tumors.
Thanks for the images to the clinical pathology service of the Spanish hospital in Mexico City.
The authors declare that there is no conflicts of interest.
None.

©2023 Elias, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.