eISSN: 2469-2778


Research Article Volume 9 Issue 6
1Department of Zoology, Faculty of Science, Zawia University, Libya
2Department of Physiology, Faculty of Medicine, Sabratha University, Libya
3Department of Zoology, Faculty of Science, Sabratha University, Libya
Correspondence: Azab Elsayed Azab, Department of Physiology, Faculty of Medicine, Sabratha, University, Libya
Received: November 19, 2021 | Published: December 30, 2021
Citation: : Abushofa FA, Azab AE, Ghawi HM. Assessment of the haematological alterations in cervical cancer patients attending sabratha national cancer institute, Western Libya. Hematol Transfus Int J. 2021;9(6):125-132. DOI: 10.15406/htij.2021.09.00269
Background: Cervix cancer is one of the most common cancers in women worldwide, and is the third most common malignant disease in women. It is one of the main health problems in Libyan women. Blood act as a pathological reflector of the status of exposed patient to infections and other conditions. Laboratory tests on the blood are vital tools that help detect any deviation from normal in the human body As the disease progresses, changes appear in haematological parameters which have been of relevant consideration in context of cancer patients.
Objectives: The present study aimed to evaluate the alterations in haematological parameters among cervical cancer patients in Sabratha National Cancer Institute.
Subjects and methods: The present study was conducted on 150 cervical cancer patients, attending the National Cancer Institute of Sabratha from the 11th February, 2006 to the 3rd February, 2020. This study was approved by the Research and Ethical Committee of Sabratha University and Sabratha National Cancer Institute. Age was extracted from patient files. Also, 60 healthy individuals without any chronic disease were recruited for the control group. Blood samples were collected by vein puncture, 3 ml of venous blood withdrawn from each participant in the study by using disposable syringes under aseptic technique; they then transferred to a sterile EDTA tube, for complete blood count. The statistical significance of differences between groups was evaluated with the Mann Whitney U test.
Results: The results showed that the mean age of the cervix cancer patients was 53.37±11.6 years. RBCs count, hemoglobin concentration, Hct value, MCH, MCHC, and lymphocytes % were significantly (P<0.01) decreased compared with the healthy individuals. On the other hand, leukocytes and platelets count, mixed %, neutrophils %, PLTs/Lymph, and Neutrophils/Lymphocytes ratios were significantly increased as compared with the healthy individuals.
Conclusion: It can be concluded that a significant increase in leukocytes and platelets count, mixed %, neutrophils %, and the studied inflammation related haematological parameters and a significant decreased in lymphocytes %, RBCs count and most its indices. Further haematological studies are needed to confirm these results. Also, there is need to routinely monitor the haematological parameters and among cervical cancer patients.
Keywords: Cervix cancer, CBC, Haematological parameters, Sabratha National Cancer Institute, Western Libya
CBC, complete blood count; HPV, human papillomavirus; IQR, interquartile range; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; NLR, neutrophil-to-lymphocyte ratio; Neut/Lymph, Neutrophils/Lymphocytes Ratio, PLR: platelet-to-lymphocyte ratio; PLTs/Lymph, platelets/lymphocytes ratio; RBCs, red blood corpuscles; WBCs, white blood cells.
Cancer is a group of diseases that can be induced as a result of abnormal uncontrolled division of cells in the human body. Epidemiologically, cancer is the second cause related to death worldwide after heart disease.1 Among all human malignancy, cervix cancer is one of the most common cancers in women worldwide,2 and is the third most common malignant disease in women. The incidence and mortality rate of cervical cancer are more prevalent in sub-Saharan Africa, Southeast Asia, Latin America, the Caribbean, Central and Eastern Europe, Zimbabwe, Malawi, and Uganda. Whereas, it is less prevalent in Western Asia.1 It is estimated that the highest rate of incidence of cervical cancer: in Five countries including India, China, Indonesia, Brazil, and the Russian Federation.4 Cervical cancer is one of the main problems in Libya.5 It has been found that it is the second most common tumor among females in Algeria and Morocco and the third most common tumor in Tunisia. Due to the lack of cancer screening and prevention programs in Libya, only a few cases have been reported in the eastern region of Libya. Almost 100% of all cervical cancer cases are caused by human papillomavirus which ranks as the most sexually transmitted infection worldwide.6
The Cervix is the lower part of the uterus that connects the uterus with the vagina. It is divided into two portions endocervix is covered by glandular columnar cells and ectocervix is covered by squamous cells. Almost all cases of cervical carcinoma originate in the transformation zone from the ecto- or endocervical mucosa. The transformation zone is the area of the cervix between the old and new squamocolumnar junction.7 According to data available until 2013, the highest prevalence of human papillomavirus (HPV) (14.6%) in Tunisia for unknown reasons.8 The prevalence of HPV in the population of western Algeria was 40% in vaginal cancers, 17% in vulvar cancers, and 33% in anal cancers.9 The most cases of cervical cancer occur as a result of HPV- 16 and HPV- 18. High-risk types, especially HPV 16, are found to be highly prevalent in human populations.7 whereas infection by others causes warts and benign lesions and is considered low risk (types HPV- 6 and HPV- 11).10
Features of early invasive cancers of the cervix produce few symptoms in premenopausal women as vaginal bleeding after intercourse, irregular vaginal bleeding, and watery vaginal discharge, severe pelvic pain caused by tumor metastasis in bone.11 The incidence rate of uterine cancer increases in young adults.7 risk factors for infection appear in women who have their first pregnancy at an early age, progesterone-based contraceptives.
Progesterone is considered to be a stimulator of HPV gene expression. Multiple sexual partners, those who have children from a young age, and sexual behavior, vitamin A, and C deficiency, and smoking are also considered as risk factors.12,13 Blood act as a pathological reflector of the status of exposed patient to infections and other conditions. Laboratory tests on the blood are vital tools that help detect any deviation from normal in the human body.14As the disease progresses, changes appear in haematological parameters such as WBCs, RBCs, haemoglobin which have been of relevant consideration in context of cancer patients.15 Cervical cancer has direct impact on the haematological parameters.16 Leukocytosis has been evaluated in many studies in lung cancers and colorectal cancer.17,18 The association between leukocytosis and the stage of cervical cancer was demonstrated in another study.19
In the view of the limited availability of the extent of cervical cancer in Libya, especially in the western region, and the lack of studies published at the present time on the haematological changes in cervix cancer patients. So, the present study aims to evaluate the alterations in haematological parameters among cervical cancer patients in Sabratha National Cancer Institute.
Study design and population
The present study was conducted on 150 cervical cancer patients, attending the National Cancer Institute of Sabratha for a Pap Smear screening to detect cancerous or pre-cancerous conditions of the cervix or other medical conditions from the 11th February, 2006 to the 3rd February, 2020, were enrolled in this prospective study. This study was approved by the Research and Ethical Committee of Sabratha University and Sabratha National Cancer Institute. Age was extracted from patient files. Also, 60 healthy individuals without any chronic disease were recruited for the control group. Blood samples were collected by vein puncture, 5 ml of venous blood withdrawn from each participant in the study by using disposable syringes under aseptic technique; they then transferred to a sterile EDTA tube, for complete blood count.
Determination of haematological parameters
Red blood cells count, haemoglobin concentration, hematocrit value, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, white blood cells count, differential count of leucocytes, and blood platelets count were determine using an automated haematology analyzer Sysmex (KX 21) machine in Sabratha Isolation Centre laboratory.
Statistical analysis
Continuous variables were presented as medians (interquartile range [IQR]) The data were analyzed using Graph Pad Prism software version 7. The Kolmogorov-Smirnov test was used to assess the normality of distribution of continuous variables. The statistical significance of differences between groups was evaluated with the Mann Whitney U test. P-value of <0.05 was used to establish statistical significance.
The mean age of the cervix cancer patients included in the current study was 53.37±11.6 years (26–88 years). The results in Table 1 & Figures 1–3,5,6 show that patients with cervix cancer had a significant (P< 0.01) decrease in the median (IQR) of RBCs count [(x 106/μL), 3.74 (3.33-4.17)], hemoglobin concentration [(10.05 (9.225-11.18) g/dl], Hct value [30.80(26.83-33.40)], MCH [27.90(25.83-29.40) Pg], and MCHC [33.00(31.50-34.33) g/dl] compared with the healthy individuals (median (IQR) 4.11 (3.90-4.29), 12.90(12.50-13.60), 36.30(35.25 -37.88), 29.00(28.05-30.00), 34.70(34.00-35.43), respectively. Data in Table 1 & Figure 4 show a none significant (P>0.05) change in median (IQR) of mean corpuscular volume (MCV) in cervix cancer patients [83.65(77.70-87.30) μ3] compared with the healthy individuals [83.00(81.00-84.15) μ3].
|
Groups |
Control |
Cervix cancer patients |
Mann Whitney test |
P Value (Summary) |
|
Parameters |
Median (IQR) |
Median (IQR) |
||
|
RBCs count (x 106/μL) |
4.11 (3.90-4.29) |
3.74 (3.33-4.17) |
2737 |
< 0.0001 (***) |
|
Hemoglobin (g/dl) |
12.90(12.50-13.60) |
10.05 (9.225-11.18) |
471.5 |
< 0.0001 (***) |
|
Hct (%) |
36.30(35.25 -37.88) |
30.80(26.83-33.40) |
915.5 |
< 0.0001 (***) |
|
MCV (μ3) |
83.00(81.00-84.15) |
83.65(77.70-87.30) |
4393 |
0.9048 (n) |
|
MCH (Pg) |
29.00(28.05-30.00) |
27.90(25.83-29.40) |
3046 |
0.0003 (***) |
|
MCHC (g/dl) |
34.70(34.00-35.43) |
33.00(31.50-34.33) |
2129 |
< 0.0001 (***) |
Table 1 Median (IQR) of erythrocytes count and its indices in control and cervix cancer patients
IQR, Interquartile range; ns, none significant difference compared with the controls , *: significant difference compared with the controls at (P<0.05). (**) significant difference compared with the controls at (P<0.01), (***) significant difference compared with the controls at (P<0.001).
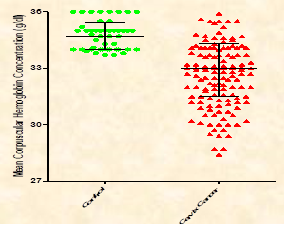
Figure 6 Median (IQR) of mean corpuscular hemoglobin concentration in controls and cervix cancer patients.
The results in Table 2 and Figures 7–12 show that patients with cervical cancer had a significant (P<0.01) increase in the median (IQR) of leukocytes count (x 103/μL) [9.07(7.08-13.30)], Mixed [,7.40(5-10)], neutrophils % [78.80(64.83-84.40)], Platelets Count (x103/μL) [268.0(200-368)], PLTs/Lymph ratio [202.3(140-346.2)], and Neutrophils/Lymphocytes ratio [5.510(2.43-10.10)], respectively compared with the healthy individuals median (IQR), 7.1 (6.6-7.475), 6 (5-7), 63 (62-66.75), 214 (201-291.8), 108 (97.10-140.7) and 2.1(1.9-2.475), respectively. Conversely, the median (IQR) of lymphocytes % in cervix cancer patients was significantly (P< 0.0001) decreased 13.75(8.125-25.60 when compared with the healthy individuals 30.00(27.13-32) (Table 2 & Figure 13).
|
Groups |
Control |
Cervix cancer patients |
Mann Whitney test |
P Value (Summary) |
|
Parameters |
Median (IQR) |
Median (IQR) |
||
|
WBCs count (x 103/μL) |
7.100(6.600-7.475) |
9.07(7.08-13.30) |
2314 |
< 0.0001 |
|
Lymphocytes % |
30.00(27.13-32.00) |
13.75(8.125-25.60) |
1464 |
< 0.0001 |
|
Mixed % |
6 (5-7) |
7.40(5-10) |
3149 |
0.0008 |
|
Neutrophils % |
63.00(62.00-66.75) |
78.80(64.83-84.40) |
2019 |
< 0.0001 |
|
Platelets Count (x103/μL) |
214.0(201.0-291.8) |
268.0(200.0-368.0) |
3587 |
0.0257 |
|
PLTs/Lymph ratio |
108.0(97.10-140.7) |
202.3(140.0-346.2) |
1444 |
< 0.0001 |
|
Neut/Lymph Ratio |
2.100(1.900-2.475) |
5.510(2.430-10.10) |
1582 |
< 0.0001 |
Table 2 Median (IQR) of Leukocytes and platelets count, differential count of leukocytes, PLR, and NLR in control and cervix cancer patients
IQR, Interquartile range; ns, none significant difference compared with the controls , *, significant difference compared with the controls at (P<0.05). (**) significant difference compared with the controls at (P<0.01), (***) significant difference compared with the controls at (P<0.001).
The results of the current study showed that the mean age of the cervix cancer patients was 53.37±11.6 years. Similar results were recorded by El Mistiri et al.20 who reported that the median age of incidence of cervix cancer in eastern Libya was 50 years. Wang et al.21 reported that the median age of the cervix cancer patients was 51 years (range, 25- 79 years). Also, Okwesili et al.14 mention that the majority of the cervical cancer subjects were in the 51-60 years of age group. In addition, Gascon and Barret-Lee,22 2006 reported that 70% of cervical cancer in Nigeria was seen between 26-50 years with peak age range of 34-45 years.
Anemia, leukocytosis, and markers of inflammation such as neutrophilia23,24 and elevated neutrophil-to-lymphocyte ratio (NLR)19,24,25,26 are most notably is tumour related as a poor prognostic marker in cervical cancer.19,23,24,26–29
The present study showed that RBCs count, hemoglobin concentration, Hct value, MCH, MCHC, and lymphocytes % were significantly (P<0.01) decreased compared with the healthy individuals. Finding from the present study is run parallel to a previous study which indicated that a significant decrease in the RBCs count, and hemoglobin content compared with the healthy volunteers.15 Wang et al.21 reported that hemoglobin concentration was significantly decreased compared with the controls. Okwesili et al.14 found that the mean Hct value was significantly (P<0.05) lower among cervical cancer patients compared to the healthy subjects. Also, the study of Gascon and Barret-Lee,22 2006 showed that red cell indices, MCH, and MCV were lower among cervical cancer patients compared to controls. Al-Araji, and Hamad30 reported that the hemoglobin concentration and hematocrit value were decreased in the patients with carcinoma of the uterine cervix compared with normal ranges. indicating that the patients have sever degree of anemia, that may be due to progressive bleeding as intermenstrual bleeding after intercourse as spontaneous. Decreased Hb level was reported in cervix cancer patients.31–33
Iron deficiency and tumor bleeding are common causes of anemia in cervical cancer.34 Anaemia seen in cervical cancer has the characteristics of anaemia of chronic disorder associated with low PCV. Several factors may be responsible for the high prevalence of anaemia seen among cervical cancer patients, may be due to lower socioeconomic status and poor nutrition of the former, haemorrhage associate with iron deficiency, anorexia associated with cancers generally can also be associated with nutritional anaemia seen in these cases, metastasis to the bone marrow from cervical cancer can be associated with suppression of erythropoiesis and infection in fungating malignancies may be associated with red blood cell haemolysis and leukocytosis.14 Also, the Nath et al.16 study revealed that most of the cervical cancer patients has decreased level of haemoglobin. Most of the patients having cervical cancer comes from low socioeconomic status and poor hygiene. This may be correlated because majority of patients of carcinoma of cervix comes from low socioeconomic background are malnourished with poor hygiene level. It has been reported that malnutrition and folate deficiency lead to suppression of immunity.16,35 Folate deficiency is responsible for decreasing immunity which might have given opportunity to HPV infection which is considered to be most important factor for causation of carcinoma of cervix. Infection will lead to increase in strengthening the defense mechanism which results in increased WBC.16,36
In the present study, leukocytes count, mixed%, and neutrophils % were significantly increased and lymphocytes % was significantly decreased compared with the healthy individuals. These results are in coincides with the study of Al-Araji, and Hamad,30 2005 who recorded that an increase in total leukocytic count in the patients with carcinoma of the uterine cervix compared with normal ranges, indicate that there are sever inflammatory reactions in the patients with uterine cervix carcinoma. The results of the study of Nath et al.16, 2015 who reported that there is a significant increase in WBCs count compared with the healthy volunteers. Wang et al., 2017 recorded that leukocytes count, neutrophils % were significantly increased and lymphocytes % was significantly decreased compared with the controls. Tumour cells secrete some chemokines, such as granulocyte-colony stimulating factor or angiotensin II, that cause an increase in neutrophil production from the bone marrow.37,38 Neoplasm of all types is associated with increase in neutrophils count. The natural killer cells are lymphocyte that are capable of destroying tumour cells without prior sensitization. However, many tumours down-regulate expression of class 1. Major histo-compatibility complex molecules as a way of evading immunity. Lymphocyte count may therefore decline.14 Also, Okwesili et al.14 recorded that the mean lymphocyte count was significantly (P<0.05) lower among cervical cancer patients compared to the healthy subjects. Some immunosuppressive factors, such as tumour growth factor b, interleukin-10 or reactive oxygen species, derived from the tumour or tumour microenvironment decreased lymphocyte production.38,39
Determination of platelet count plays a significant role in cancer management. The prognostic significance of the platelet count has been studied in several malignancies, yielding important information about clinical outcomes.14 Thrombocytosis has been associated with unfavourable prognosis or advanced disease in gynaecological and other types of cancers.40–43
The current study showed that a significant increased in the median of platelets count in cervix cancer patient as compared with the controls. This result is similar to the result of Okwesili et al.,14 who observed that the mean platelet count in the cervical cancer patients was increased compared to normal controls. Also, Wang et al., 2017 recorded that platelets count was significantly increased in cervical cancer patients compared with the controls. The platelet count is an additional marker of systemic inflammation which is precipitated by the tumor formation. Proinflammatory cytokines such as IL-1 and IL-6 leads to megakaryocyte proliferation and thrombocytosis.44,45 Thrombocytosis may be seen in cancer patients as a result of cancer induced anaemia. A negative feedback effect on erythropoietic production in cases as a result of the anaemia could be for responsible for the thrombocytosis. Erythropoietin has a structural homology with thrombopoietin, although the latter is considerably larger than the former but roughly half of thrombopoietin at the N- terminal region.46
On the other hand, there was no statistically significant difference between the mean WBCs, and Platelets count, MCV, MCH, and MCHC of cervical cancer patients and the healthy subjects.14 One of the important systemic alterations is inflammation. Inflammation is a nonspecific feature of cancer and plays an important role in various aspects of cancer involving cancer initiation, promotion, progression, metastasis and clinical features.47,48 Therefore, inflammatory markers were studied in various cancer types as indicators of invasion.48,49 Inflammation plays a key role in tumorigenesis and in the progression of cervical cancer.21,50 Thrombocytosis and lymphocytopenia have been reported as a marker in host systemic inflammation.51Kose et al.48 concluded that patients with uterine cervical cancer may present with leukocytosis, increased neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. These cheap and easily available parameters, especially PLR, may provide useful information about the invasiveness of the cervical pathologies. The NLR, and PLR are markers of systemic inflammation with prognostic significance for cancers.21 The previous studies showed that a strong correlation between NLR and inflammation. Increased neutrophil% is accepted to endorse neoplastic progression, and it can repress antineoplastic properties of lymphocytes. Accordingly, NLR may be recognized as the marker of the balance between precancerous inflammatory state and cancerous immune state, and higher NLR might be indicative for tumor development.52,53 Neutrophil-to lymphocyte ratio, and platelet-to-lymphocyte ratio have been found to be associated with various kinds of cancers including non-small-cell lung cancer, pancreatic adenocarcinoma, gastric cancer, over carcinoma, renal cell carcinoma, colorectal cancers, and endometrial cancers.54–57 A growing body of evidence highlights the associations between the NLR or PLR and tumor characteristics in patients with cervix cancer. Increased NLR and PLR have been shown to be associated with stage, invasiveness, prognosis, and unfavorable histopathological characteristics of cervix cancer.48,53,58
Also, Wang et al.,21 reported that median values of NLR and PLR were higher in cervical cancer patients compared with controls and were consistently elevated during tumor progression. Increased NLR was associated with lymph node metastasis and depth of stromal infiltration, and increased PLR correlated only with LN metastasis. The pretreatment NLR or PLR value was a significant predictor of LN metastasis, which enhanced when NLR and PLR values were combined. Further, NLR and PLR were as effective for predicting distant tumor metastasis. Authors suggested that pretreatment values of NLR and PLR might be helpful to predict the presence of distant and LN metastasis in patients with cervical carcinoma, but not adequate prognostic factors for early-stage patients.
The current study showed that the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios were significantly higher in cervical cancer patients compared with the healthy individuals. These results are run parallel with the result of Tas et al.53 2019 who found that the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios were significantly higher in patients with cervix cancer than in controls. Authors concluded that, in addition to age of patients, and determination of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios, that are simple, low-cost, and readily available markers of systemic inflammation, may help in decision making precancerous pathologies of the cervix.
NLR was defined as a potential marker to determine inflammation in systemic disease.17,48,49,59–61 However, it is well known that inflammation plays an important role in various aspects of cancer. A correlation between the increase of NLR and endometrial cancer invasiveness has been demonstrated.48,49
In the current study, NLR was higher in the cervical cancer group compared to the controls. Similarly, there was an association between NLR and cervical cancer invasiveness.19,48 It was emphasized that PLR is a novel marker for inflammation, which incorporates both hematological factors.51 The platelet-lymphocyte ratio is an inflammatory marker and has been studied in uterine cancer and uterine cancer precursor lesions.48,49 Furthermore, there was an association and correlation between cervical malignant lesions and PLR in the present study. Because of this, the researchers believe that PLR may be a suitable marker like NLR. The main drawback to the current study was its retrospective nature and small sample size.48 Increased NLR and PLR as a result of increased neutrophil and platelet counts may contribute to the stimulation of cancer development in patients with precancerous cervical pathologies.53
It can be concluded that a significant increase in leukocytes and platelets count, mixed %, neutrophils %, and the studied inflammation related haematological parameters and a significant decreased in lymphocytes %, RBCs count and most its indices. Further haematological studies are needed to confirm these results. Also, there is need to routinely monitor the haematological parameters and among cervical cancer patients.
None.
The authors declare no conflicts of interest.

©2021 :, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.