eISSN: 2469-2778


Review Article Volume 5 Issue 2
1Department of Hematology, Kanazawa University, Japan
2Department of Internal Medicine, Sohag University, Egypt
Correspondence: Mahmoud I Elbadry, Department of Hematology, Kanazawa University Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Takara-machi 13-1 Kanazawa, Ishikawa 920-8641, Japan, Tel 81-76-265-2274, Fax 81-76-234-4252
Received: July 17, 2017 | Published: September 15, 2017
Citation: Elbadry MI, Noreldin AKA, Hassanein HA. After moving of regulatory T-cell therapy to the clinic: will we need a new Tregs source?. Hematol Transfus Int J. 2017;5(2):224-230. DOI: 10.15406/htij.2017.05.00117
The last few decades, an intense interest has been developed in using regulatory T cells (Tregs) for immunotherapy by the biomedical community, depending on the facts that Tregs can modulate both innate and adaptive immunity. Recently, the therapeutic potential of Tregs has been moved to clinical practices in the field of autoimmune diseases and after allogeneic transplantation. Many clinical trials have involved Treg adoptive transfer to treat autoimmune diseases, solid organ transplantation, and hematopoietic stem cell transplantation (HSCT). The researchers have designed many strategies to isolate, preserve, expand, and infuse Tregs. However, the sources of Tregs cells remain one of many obstacles hindering Treg clinical applications. Here, we review current approaches have being explored for Treg expansion and the possible sources including induced pluripotent stem cells (iPSCs) in the perspective of clinical therapeutic protocols.
Keywords: immunotherapy, regulatory T cells, allogeneic transplantation, lymphocyte, GITR
AMP, adenosine monophosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; IDO, indoleamine 2,3-dioxygenase; IFN, interferon; IL, interleukin; LAG3, lymphocyte-activation gene 3; TCR, T cell receptor; TGF-β: transforming growth factor-β, Th, T helper cell; HSCT, hematopoietic stem cell transplantation; Tregs, regulatory T cells; iPSCs, induced pluripotent stem cells; SOT, solid organ transplantation; NKT, natural killer T; TNF, tumour-necrosis factor; CTLA4, cytotoxic T-lymphocyte antigen 4; FOXP3, forkhead box P3; AA, aplastic anemia; SLE, systemic lupus erythematosus; T1D, type 1 diabetes; DCs, dendritic cells; PB, peripheral blood; UCB, umbilical cord blood; FACS, fluorescence activated cell sorting; TSDR, treg-specific demethylated region; CAR, chimeric antigen receptors; EAE, experimental autoimmune encephalitis; ES, embryonic stem; ATG, anti-thymocyte globulin
One of the most attracting attention features of the immune system is its capacity to produce a highly specific response. However, autoimmunity and alloimmunity protect the host against malignancy and infection, the unrestrained immune system activation leads to clinical disorders like autoimmune diseases. Autoimmune diseases are defined as a condition arising from an abnormal immune response which target the body’s own healthy tissues by mistake. As, the breakdown of mechanisms of self and non-self-recognition by the immune system is the main cause of these diseases. Furthermore, induction of immunologic tolerance is essential to improving outcomes in diseases characterized by immune system activation: autoimmune disease,1 hematopoietic stem cell transplantation (HSCT)2 and solid organ transplantation (SOT).3 Treatment options for autoimmunity as chemical agents have shown to be beneficial in autoimmunity, but they can result in increasing the susceptibility of patients to life threatening opportunistic infections. Identification of newer therapies methods which could specifically block auto-inflammatory immune cells while allow for an intact immune response to other antigens are considered a long-sought goal in hematology research. Foxp3-expressing regulatory T cells (Tregs) play a crucial role in maintaining peripheral tolerance, homeostasis and preventing autoimmunity by suppressing abundant immune system activation and promote immunologic tolerance. Tregs cells are highly antigen specific and highly versatile cells which can control responses of various immune cells including conventional CD4+ T cells, CD8+ T cells, natural killer T (NKT) cells, NK cells, B cells, and various antigen-presenting cells as Tregs cells have many different immunosuppressive mechanisms and strategies in response to the tissue microenvironment.4 Tregs represent a peripheral system to maintain self-tolerance and prevent over-stimulation of immune responses and “smart” therapeutic agents. Here, we try to review the possible roles of Tregs therapy in autoimmune diseases, solid organ transplantation, and hematopoietic stem cell transplantation (HSCT) and highlight the current approaches which have being explored for Treg expansion and the possible sources including induced pluripotent stem cells (iPSCs) in the perspective of clinical therapeutic protocols as a source with the potential to cure these disorders.
Treg-cell
Treg cells are CD4+CD25+ regulatory T cells, constitute about 1-3% of circulating CD4+ T cells in the periphery which develop early during lymphocyte maturation in the thymus and use a diverse T-cell receptor (TCR) repertoire (Figure 1A). Deletion of self-reactive T cells in thymus is called central tolerance; in the other side Tregs represent a peripheral system to prevent over-reactive immune system and maintain self-tolerance (Figure 1B). Tregs express several cell-surface characteristic molecules including CD28, CD62L, GITR (glucocorticoid-induced tumor-necrosis factor (TNF)-receptor-related protein) and CTLA4 (cytotoxic T-lymphocyte antigen 4) as well as a specific transcription factor, FOXP3 (forkhead box P3), which controls the development and suppressor function of these 24cells. Foxp3-mutant mice show an evidence of FOXP3 importance as these mice develop a severe lymphoproliferative autoimmune syndrome as a result of a Treg cell deficiency.5,6
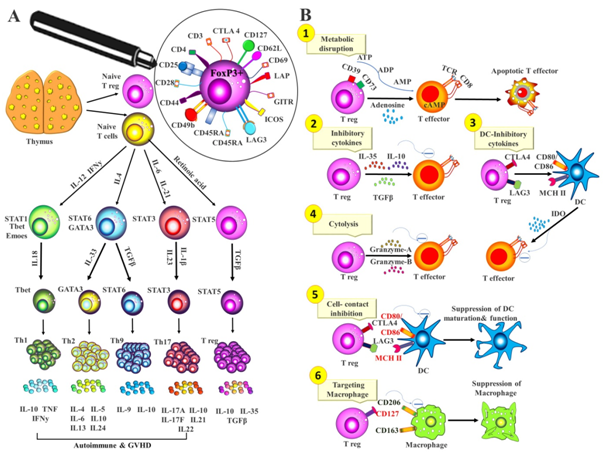
Figure 1 Key markers of functional CD4+FoxP3+ regulatory T cell.
Regulatory T-cell therapy in autoimmune diseases and transplantation
Reduced numbers of Tregs have been reported in patients with autoimmune diseases like, juvenile idiopathic arthritis,7 psoriatic arthritis,8 aplastic anemia (AA),9,10 systemic lupus erythematosus (SLE),11 and autoimmune liver disease.12 Also Tregs have been shown to be defective in Type 1 diabetes (T1D).13 It is worth mentioning that the lower levels of circulating Treg cells correlate with poorer prognosis and a higher autoimmune disease activity.7,14 It is plausible that the administration of ex vivo-differentiated FoxP3(+)Tregs or tolerogenic dendritic cells (DCs) that promote Treg differentiation could be a potential therapy for these autoimmune diseases. This type of treatment was successful in treating Type 1 diabetes (T1D). Bluestone et al.13 reported on a phase 1 trial which was designed to assess safety of Treg adoptive immunotherapy in T1D of fourteen adult subjects with T1D received ex vivo-expanded autologous CD4(+)CD127(lo/-)CD25(+) polyclonal Tregs. This study showed there were no infusion reactions or Tregs cell therapy-related high-grade adverse events and transient increases in Tregs in recipients also this study showed that retained a broad Treg FOXP3(+)CD4(+)CD25(hi)CD127(lo) phenotype long-term up to 25% of the peak level remained in the circulation at 1 year after transfer.13 C-peptide levels persisted out to 2+years after transfer in several individuals. These results support the development of a phase II trial to test efficacy of the Treg therapy in new onset T1DM,15 as well as studying the efficacy in other autoimmune diseases.
Various types of T cells have been shown to contribute to transplant tolerance. These include the Tregs that express the transcription factor FOXP3, IL-10-producing Tr1 cells, CD8+28- T cells, and anergic T cells.16 Tregs cells were successful in treating established chronic graft-versus-host disease (cGVHD) in a mouse model with multi-organ-system diseases.17 Also, Pierini et al.17 showed that allogeneic cell therapy with NK or Tregs improved HSC engraftment and a potential effectiveness of the cell therapy for ameliorating GVHD and for accelerating immune reconstitution after hematopoietic stem cell transplantation.17 However, many limitations of using regulatory T-cell therapy in solid organ transplantation as Treg may not be sufficient to stand-alone as monotherapy for solid organ transplantation in humans. Investigators have suggested to overcome the possible hurdles using combination therapy with immunomodulatory drugs which could possibly improve the in vivo efficacy of Treg.16
The ONE Study a Phase I/IIa clinical trial was designed to test the safety and practicality of seven different regulatory cell populations in living donor kidney transplantation and currently under way.18 The administration of iTregs generated in vitro to 10patients undergoing living donor liver transplantation has been reported.4 This iTregs were generated by co-culturing irradiated donor PBMCs with recipient PBMCs in the presence of costimulatory blockade. This study showed that administration of iTregs was safe and appeared to facilitate early weaning and discontinuation of immunosuppression in 5 of the 10patients. However, these findings are exciting, longer-term follow-up in these patients was essential. In another long-standing study, the adoptive transfer of an ex vivo-generated regulatory T-cell-enriched cell product for 10 consecutive adult patients early post-liver transplantation succeeded in weaning and cessation of immunosuppressive agents in seven patients, the other 3 recipients with autoimmune liver diseases were low-dose immunotherapy dependence. Infusion of these cells caused no significant adverse events. Interestingly, all patients at the last follow up were well with normal graft function and histology and some of them have been drug free for more than 24months. This results support the role of T regs cell in drug minimization and operational tolerance induction in living donor liver recipients without autoimmune liver diseases.19
Sources and manufacturing of treg cells
Many ex vivo strategies have been designed to isolate, preserve, expand, and infuse Tregs.20 Also in vivo protocols to manipulate Treg populations have been considered (Figure 2).21
Non-specifically treg cell expansion
Banked umbilical cord blood (UCB) and an adult peripheral blood (PB) may serve as a Treg cells source. An adult PB apheresis unit can yield on the order of 108 Tregs cells in comparison to frozen UCB unit which can yield approximately 5-7.5×106Tregs. Multiple studies have reported the expansion of nTregs from peripheral blood (PB) and umbilical cord blood (UCB),19,22 but the successful isolation process requires labeling cell surface markers with a tagged antibody and cell sorting via fluorescence-activated cell sorting (FACS) or separation by magnetic bead coated with CD3- and CD28-specific antibodies, using high doses of IL-2 that have been used for expansion of non-specifically Treg cells. Regrettably, FOXP3 as one of the most important marker of Tregs requires cell permeabilization for detection, which renders cells unusable for adoptive transfer. Also, activated CD4+ conventional T cells may transiently express CD25, patterns of CD127 (the IL-7 receptor α-chain).23 Evaluation of the expanded Treg cells surface expression markers show maintained cell-surface expression of multiple Treg-cell markers, including CD25, CD62L, HLA-DR, GITR, CTLA4 and they maintain intracellular expression of FOXP3. Function evaluation of the expanded Treg cells prove that these cells maintain regulatory suppressor function and cytokine secretion, these cells produce less IL-2 and interferon-gamma and more IL-10 and TGF-beta than conventional CD4+CD25- T cells that are expanded in vitro in a similar manner.
Recently, Brunstein et al.24 studied the safety and clinical outcomes of patients treated with UCB -derived Tregs that expanded in cultures stimulated with K562 cells modified to express the high-affinity Fc receptor (CD64) and CD86, the natural ligand of CD28 (KT64/86). They treated eleven patients with Treg doses from 3-100×106Treg/kg. The median proportion of CD4(+) FoxP3(+) CD127(-) in the infused product was 87%. This study showed that KT64/86-expanded UCB Tregs were safe and resulted in low risk of acute GVHD as the incidence of grade II-IV acute GVHD at 100 days was 9% vs 45% in controls also; chronic GVHD at 1 year was zero in Tregs and 14% in controls.24 Many investigators used polyclonal Treg expansion as a source for Treg.25 However, the cellular and molecular basis for Treg destabilization during in vitro stimulation is still unclear and still work as limitation in Treg therapy as the loss of FOXP3 is likely owing to destabilization of FOXP3 expression in Tregs instead of outgrowth of a few contaminating conventional T cells. So, we still need to determine an optimized expansion protocol to maximize yield without compromising purity.
Alloantigen-reactive Treg cell expansion
Another studies reported that 5-32times less alloantigen-expanded Tregs may be sufficient to achieve the same therapeutic efficacy as polyclonal Tregs.26 However, more efforts should be made to expand human indirect Tregs as most successes in expanding human alloantigen-reactive Tregs have been in generating direct Tregs. Several approaches have been developed to increase the suppressive activity of the marked allogeneic polyclonal regulatory T cells cross-reactivity. One study showed that chronic activation of human CD4+ T cells by autologous APCs in the presence of IL-10 and type I interferon’s gives rise to CD4+ TR1 clones that produce high levels of IL-10. These cells suppressed the in vitro alloantigen-specific proliferation of CD4+ T cells. Another study16 showed that the presence of TGF- in T cells primed developed into contact-dependent and cytokine-producing regulatory T cells. Many researchers suggest that these approaches are ready to adapt for the clinic. Previously Trzonkowski et al.27 described first-in-man clinical effects of adoptive transfer of ex vivo expanded CD4+CD25+CD127- T regulatory cells (Tregs) in the treatment of GVHD, the isolated cells were cultured for a maximum of 3 weeks with 10% of complement-inactivated autologous fresh plasma from auto transfusion with high concentration of interleukin 2 and anti-CD3/anti-CD28 beads in 1:2 ratio.27 The hematology society still waits a phase I trial of BMT with administration of TR1 cells that have been expanded ex vivo using IL-10 and type I interferons.
Induction or engineer of Tregs cell ex vivo and in vivo
An alternative strategy to ex vivo naïve Treg isolation and expansion is ex vivo conversion of CD4+CD25- naïve T cells into iTregs with suppressor function.20 It has been proposed that conventional CD4+ T cells can be converted to Tregs during ex vivo expansion with the addition of TGF-β together with rapamycin or all-trans retinoic acid.28 Although FOXP3 is a marker for activated human Tregs human, FOXP3 expression alone does not distinguish activated T cells from Tregs as Tregs transiently express FOXP3. The DNA methylation status of the Treg-specific demethylated region (TSDR) is another marker that can be used for distinction between ex vivo-isolated Tregs and in vitro induced Tregs, which is important for Treg commitment and stability. Many authors reported that ex vivo-isolated Tregs have a demethylated TSDR, whereas in vitro-induced Tregs have fully methylated TSDR suggesting that they are not committed Tregs.28,29 Stabilization of induced Tregs can be achieved by inhibiting or knocking down the DNA methyl transferase promotes demethylation of the FOXP3 locus.30,31 However, many experiments are needed to confirm the evidence on the commitment and stability of ex vivo induced Tregs before they can be considered as a viable source of therapeutic Tregs for humans.
Taking advantage of the growing field of Chimeric Antigen Receptors (CAR) for gene-modified Tregs and generation Tregs by T cell receptor gene transfer opens an alternative approach to engineer Tregs with the ability to target specific antigens by expressing antigen-specific TCRs.32,33 Several animals studies support that CAR-expressing Tregs can be efficacious in preventing experimental autoimmune encephalitis (EAE)34 or murine colitis and its associated cancer35 or may be in the future will support the efficacy of CAR-expressing Tregs in the setting of transplantation. According to in vivo expansion of nTregs, many variety of strategies used to induce Treg number or potency in vivo including expansion of nTregs and conversion of non-Tregs to iTregs like, prevention of allograft rejection in mice by treating mice prior to allografting with a donor alloantigen and a non-depleting anti-CD4 antibody which achieves Treg expansion.21 As well as, adoptive transfer of Tregs isolated from treated animals revoked rejection,36 nTregs isolated from naïve animals may also prevent rejection, But the remaining obstacle represented in the long-term allograft survival requires 10-fold more Tregs compared with Tregs isolated from tolerant animal treated with antigen exposure alone.37
Although, we summarized many approaches can be achieved by expansion of CD4+CD25+ Treg cells ex vivo or induction of Treg cells in vivo which is now well established in animal models,38 significant challenges remain to bring the use of expansion of Treg into the clinic. Many researchers try to identify the obstacles hindering Treg clinical applications to found possible solutions.39 One of these limitations is in generating a large number of Treg cells which essentially required to perform the cell-based therapies, in spite of the growing number of methods for isolating Treg cells, a more comprehensive Treg cell subset increases the contamination by the non-regulatory teffs. A minor population of Foxp3(+) cells loses Foxp3 expression over time; these “ex-Foxp3” cells may display an activated conventional T cell phenotype and become pathogenic in vivo. Tregs that lose FoxP3 can develop an effector memory phenotype and become pathogenic in animal models.40 No approach until now has been established to isolate the population of Treg cells with 100% specificity and the resistance of Treg cells to exogenous expansion is another limitation.41 Also, the survival of Treg cells is another critical factor in Treg cell-based therapies. In Absence of certain cytokines (e.g., IL-2) and other cells, Treg cells are organized to apoptosis.42 Bcl-xL is an anti-apoptotic protein that can sustain the survival of T lymphocytes and because of the low expression of the bcl-2 family proteins; Treg cells are prone to apoptosis.42
Induced pluripotent stem cell derived -Tregs cells
In 2006, Yamanaka showed that mouse embryonic and adult fibroblasts acquire properties similar to those of embryonic stem (ES) cells after retrovirally introducing genes encoding four transcription factors, namely Oct3/4, Sox2, Klf4, and c-Myc.43 They called these cells induced pluripotent stem cells (iPSCs). iPSCs have high potential for advancing the field of T reg cell-based therapies, because of the plasticity and the potential for an unlimited capacity for self-renewal of this cell. iPSCs technology enabled generation of DCs or Tregs derived from iPSC-HPSCs in vitro which can be activated by IL2, TNF-α.44 Functionally active DC regs were recently generated from murine iPSCs. These cells showed normal functional properties in both in vitro and in vivo as well as the ability to generate Tregs in vitro.45 It was reported that programming of functional CD4+ Treg cells or CD8+ cytotoxic T lymphocytes from iPSCs can be used for adoptive immunotherapy of autoimmune arthritis and cancers.46,47 However, caution is needed when translating in vitro induction of Treg cells to in vivo application. A number of strategies have been suggested to regulate the numbers and functionality of Treg cells such as ectopic expression or the acetylation modulation of Foxp3.48 But, it seems apparent that using iPSC derived Treg cells for each patient is expensive and takes a long time as well as administering autologous PB Tregs. Assessment of the cost-effectiveness of using Tregs will ultimately be required. In the ONE Study, the authors estimation for the cost of administering autologous PB Tregs was about £30 000 (US$45 000), which was over and above the cost of maintenance immunosuppression.49
Haplobank of iPSC lines homozygous for a range of HLA types should be the best solution to this problem as will simplify HLA matching and could extend iPSC-derived therapies beyond the autologous setting using a custom-made. It should be executable according to previously reported estimation, for United Kingdom population,50 Japanese population51 and California population.52 However, new methods for expanding polyclonal and antigen-specific regulatory T cells should design in the future. This highlight directs us to answer important question, Can Treg cell therapy stand alone in treatment of autoimmune and GVHD or need combination with immunosuppression drugs. Several studies showed that the initial confidence in adoptive Treg cell therapy as a self-sufficient entity, another experimental data have shown that preparation of in vivo environment and encourage cell engraftment and increasing the chance of tolerance induction and enhance the efficacy of Treg therapy by immunosuppressive treatments or host T-cell depletion to promote long-term survival of allograft.53,54
Strategies to tailor immunosuppressive therapy to ensure the in vivo survival of the injected Tregs
Several studies have shown that the use of CNIs during adoptive Treg therapy may link with progressive decline in Treg numbers,55 fail to support the differentiation of the highly suppressive CD4+CD25+CD27+ Treg subset upon alloantigen stimulation,56 reduce FOXP3 expression in nTregs,57,58 and diminishes the frequencies of CD4+CD25+FOXP3+ T cells.59 On the other hand, MMF and low-dose tacrolimus had an induction of CD4+CD25+FOXP3+ Tregs as showed in kidney transplanted recipients treated with these drugs that permit Tregs expansion in the periphery and accumulation in the allograft and the maintenance of their suppressive function which was confirmed by in vitro analysis of these cells.60 Other studies showed that MMF has no effect on Tregs numbers and function but may facilitate the induction of a more tolerogenic environment.58 Many researchers described the positive effects of dexamethasone and prednisolone on expansion of Tregs by the IL-2-dependent expansion of FOXP3+CD4+CD25+ T cells61 or maturation of Tregs by increasing FOXP3 expression,62 and restore the impaired suppressive function of Tregs.63 The suggesting strategies to create a tolerogenic environment to ensure the in vivo survival of the injected Tregs or enhance their longevity in vivo by tailoring this clinical immunosuppressive therapy protocol; one month prior to Treg infusion, in parallel with low-dose tacrolimus, the patients are given rapamycin, to promote selective Treg expansion in vivo,64 beside Treg-supportive immunosuppressive regimen including anti-thymocyte globulin (ATG), to induce lymphopenia with a preferential preservation of Tregs.65 Additionally, to limit memory T cell expansion post-ATG induction, patients are started on tacrolimus and prednisolone.
Until now, many barriers to clinically feasible Treg immunotherapy on its way to resolve include Treg stability and in vivo fate, off-cell effects, and demonstration of cell preparation purity and potency, specificity and the effects of immunosuppression on Tregs. Clinical trials involving Treg adoptive transfer to treat graft versus host disease preliminarily demonstrated the safety and efficacy of Treg immunotherapy in humans. Although a minor population of true nTregs may lose FoxP3 expression, they can re-express it and remain suppressive when challenged with antigen.66 Future work will need to confirm that the obstacles overcoming truly occurred and to solve the remaining barriers for using Treg immunotherapy and establish the efficacy of specific Treg subsets for the treatment of many autoimmune diseases.
This review gives an overview of the rationale of using Treg therapy in transplantation and some autoimmune diseases, highlights the expected safety, specificity, potential efficacy, cost-effectiveness, stability and in vivo fate of Treg therapy depending on the previous experience with Treg therapy in humans, in trying to find another source for harnessing the natural immune regulatory mechanisms using Tregs cell-based therapies.
None.
No competing financial interests exist.
The author declares no conflict of interest.

©2017 Elbadry, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.