eISSN: 2469-2778


Case Report Volume 1 Issue 1
1Department of Hematology, University Hospital Vall d
2Department of Nephrology, University Hospital Vall d
3Department of Pathology, University Hospital Vall d
Correspondence: Julia Montoro, University Hospital Vall d’ Hebron, Paseo Vall de hebron 119, Barcelona, Spain, Tel 686046920
Received: February 20, 2015 | Published: April 2, 2015
Citation: Montoro J, Jatem E, Abrisqueta P, et al. Acute renal failure due to membranoproliferative glomerulonephritis and kidney tumoral infiltration in a patient with mantle cell lymphoma. Hematol Transfus Int J. 2015;1(1):12-14. DOI: 10.15406/htij.2015.01.00004
Mantle cell lymphoma (MCL) is a subtype of non-Hodgkin lymphoma (NHL) that usually presents with a disseminated disease at diagnosis. In spite that multiple extranodal sites are frequently involved, the kidney is rarely affected in MCL. In NHLs, kidney is usually involved due to direct Tumoral infiltration or to the development of Glomerulonephritis, being Membranoproliferative Glomerulonephritis (MPGN) the most common cause. Herein, we report a patient with MCL who presented at diagnosis with an acute renal failure, haematuria, and nephritic syndrome due to both MPGN and Tumoral infiltration. To our knowledge, the association between MCL with both Tumoral cell infiltration and MPGN has not been reported before. Both renal function andhaematuriaimproved after the administration of immunochemotherapyadministration for the MCL.
Keywords: non-hodgkin lymphoma, mantle cell lymphoma, proteinuria, haematuria, membranoproliferative glomerulonephritis
MCL, mantle cell lymphoma; NHL, non-hodgkin lymphomas, MPGN, membranoproliferative glomerulonephritis
Mantle cell lymphoma (MCL)represents 5-10% of all B-cell non-Hodgkin lymphomas (NHL) and is characterized by the proliferation of mature B-lymphocytes, usually co expressing CD5, that infiltrate lymphoid tissues, bone marrow, peripheral blood, and extranodal sites.1 Genetically, MCL is characterized by the t (11;14)(q13;32) translocation which leads to the rearrangement and over expression of the cyclin D1 gene.2,3 From the clinical standpoint, MCL usually has an aggressive clinical course with short responses to treatment, frequent relapses, and short survival.4
MCL is usually diagnosed at advanced stages with frequent extranodal infiltration, including peripheral blood and bone marrow, gastrointestinal tract or Waldeyer’s ring involvement.4 In contrast, urinary tract involvement in MCL is exceedingly rare. Thus, only few cases of MCL with acute renal failure, renal function impairement, and ornephrotic syndrome have been previously reported.5 In this sense, although Membranoproliferative Glomerulonephritis (MPGN) has been communicated to occur in association with lymphoproliferative disorders, only few cases of MPGN with nephritic syndrome related to MCL have been published so far. Herein we reported a patient diagnosed with MCL who developed acute kidney injury due to both MPGN and Tumoral lymphoid infiltration of the kidneys. The pathogenesis of these complications and their clinical implications are analyzed.
A 65 years-old male was diagnosed with a classic variant of MCL and referred to our institution for disease assessment and treatment. Past medical history included hypertension that was properly controlled by receiving ACE inhibitors. The patient presented with night sweats, weight loss superior to 10%, and mild fatigue that started ten months before the diagnosis. Physical examination disclosed tonsil, liver, and spleen enlargements. Initial laboratory tests showed Hb 84 g/L, MCV 97 fL, WBC count of 48.3 x 109/L with 22.7 x 109/L lymphocytes, and platelet count of 112 x 109/L. Serum creatinine and urea were of 2.8 mg/dl and 94 mg/dL, respectively, total serum protein was 74 g/L, potassium was of 6.4 mmol/l, and LDH serum was of 415 UI/L (nv< 400 U/L). The 24-hour urinary volume was 4300 mL and total proteinuria was of 2,038 mg/24 hours. Serum and urine protein electrophoresis did not revealed monoclonal component. Viral serological test were negative for HBV, HCV, HIV 1 and 2. Microscopic examination of a tonsil biopsy showed a lymphoid infiltration composed by small and medium centrocyte-like cells that were CD20+, CD5+, and cyclin D1+. The Ki 67 expression was of 20%. Cytogenetic analysis of bone marrow showed a complex karyotype {[43-44,XY,-11,der(14), t(11;14)(q13;q32),-15,der(20),t(1;20)(q24;q13.3)[7]}. Bone marrow biopsy and colon biopsy disclosed infiltration by Tumoral lymphoid cells with characteristics of MCL. Flow cytometry study of peripheral blood showed 82% of lymphocytes with MCL characteristic phenotype. Finally, PET-CT revealed multiple increased metabolic activity in several lymphoid areas, liver and spleen. Abdominal ultrasonography showed an enlarged liver of 19 cm and normal sized kidneys with increased echogenicity and multiple bilateral cysts. Altogether, the diagnosis of MCL, classical variant, stage IV-B was established.
According to local guidelines, treatment with EPOCH-R (rituximab 385 mg/m2 on day 1, etoposide 103mg/m2 on days 1-4, prednisone 130mg/m2 on days 1-5, vincristine 0.6mg/m2 on days 1-4, adriamycin 20mg/m2 on days 1-4 and cyclophosphamide 1,540mg/m2 on day 5 ), along with prophylaxis for Tumoral lysis syndrome were administered. On the fifth day after chemotherapy, the patient developed renal function impairment with macroscopic haematuria containing dysmorphic erythrocytes, increase of serum creatinine, and proteinuria up to 6.37 mg/dl and to 3.5 g/24 hours, respectively. Serum phosphates, calcium uric acid, fibrinogen, and coagulation tests were normal. Thrombosis of the renal vein or extrinsic compression by lymphadenopathy was rule out by renal ultrasonography. Flow cytometry analysis of the urine sample disclosed the presence of clonal lymphocytes with MCL phenotype. The transjugular renal biopsy examination showed mesangialhypercelullarity, thickening of capillary basal membranes, some fibrocelullar crescents (Figure 1a); moderate intimal fibrous thickening of medium and small caliber arterioles (Figure 1b); and focal intersticial infiltration by atypical lymphoid B cells cyclin D1 positive (Figure 2). The tonsil biopsy showed positive immunohistochemical staining of the cycline D1 (Figure 3).
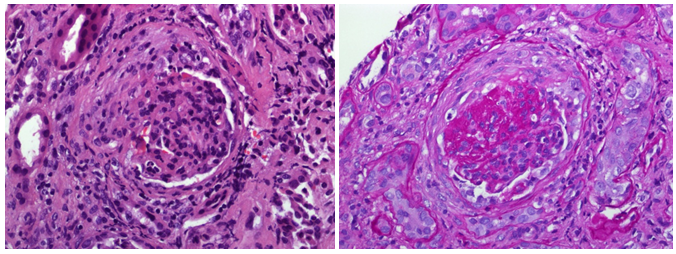
Figure 1a Mesangialhypercelullarity, thickening of capillary basal membranes, some fibrocellular crescents.
Figure 1b Moderate intimal fibrous thickening of medium and small caliber arterioles.
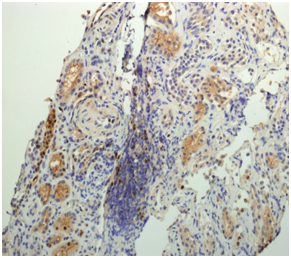
Figure 2 Focal intersticial infiltration by atypical lymphoid cells cycline D1 positive in the renal biopsy.
With all these findings, the concomitant diagnosis of both MPGN and kidney infiltration by MCL was performed. The patient was subsequently treated with high doses of intravenous methylprednisolone (1 mg/Kg/day). One week later, creatinine serum levels diminished to 2.4 mg/dl and haematuria resolved. One month after the first course of chemotherapy, peripheral blood lymphocyte count and serum creatinine rose up to the values observed at diagnosis, this being considered as an inadequate response to the therapy. For these reasons, the patient started salvage therapy with R-HAD-B regimen (rituximab 388mg/m2 on day 1 and 2, bortezomid 3,08mg/m2 on day 2 and 5, citarabine 1540mg/m2 on day 3 and dexamethasone 40mg/m2 on day 2 to 5). After four courses of such treatment the patient was considered in complete response. Moreover, serum creatinine diminished to 1.77 mg/dL, and the haematuria and proteinuria disappeared. Two months after the end of chemotherapy, the patient underwent autologous stem-cell progenitor transplant that was uneventful. Two years later the patient is still in complete remission and the renal function remains normal.
Herein, we report a patient diagnosed with MCL that developed renal insufficiency due to both kidney Tumoral infiltration and MPGN. Renal function impairment in patients with lymphoma can be due to direct causes, like tumor infiltration or ureteral obstruction, or to indirect effects such as hypercalcemia, tumor lysis syndrome, tubular obstruction by light-chain precipitation, and treatment-related effects.6 In addition to that, widespread tumour infiltration of the kidney has been reported in almost one-third of the autopsies in patients with lymphoma7 although acute renal failure due to bilateral infiltration of the kidneys is unusual and is rarely the presenting sign of a lymphoid malignancy.8,9 In our patient a direct infiltration of the kidney by lymphoid Tumoral cells with characteristics of MCL was observed. Regardless of the fact that MCL is generally diagnosed in advance stage with frequent extranodal involvement, direct Tumoral infiltration of the kidney by MCL is exceedingly rare, with few cases being reported until now.10,11 Paraneoplasticglomerulonephritishas been associated with carcinoma of the lung or the gastrointestinal tract and with Hodgkin’s lymphoma. Few cases of MPGN in patients with NHL have been described.12 MCL is rarely associated with MPGN, with only 4 cases published so far.13–15 The physiopathology of MPGN in the context of lymphoma is not well known. Chronic HCV infection is the most common cause of secondary MPGN and is often associated with cryoglobulinemia. Although HCV serology was negative in our patient, any condition associated with persistent immunological stimulation can result in secondary cryoglobulinemia.16,17 In our patient, although serum paraprotein were no detected in the laboratory tests, cryoglobulins were not directly measured and thus they cannot be excluded as a pathogenic mechanism.18,19
From the therapeutic standpoint, treatment of secondary MPGN associated with lymphoma requires both the symptomatic treatment of nephrotic syndrome and the treatment of the predisposing hematologic disorder. Of note, the remission of lymphoma parallels remission of the glomerulopathy, likewise the relapses.13 Therefore, the appearance of findings that suggest recurrence of Glomerulonephritis, should induce a work up for lymphoma relapse and vice-versa.20 According to the case herein reported, the development of renal impairment in patients with MCL should prompt physicians to exclude direct Tumoral infiltration or glomerulopathy, as these diagnoses have critical implications for the treatment and follow-up of these patient.
None.
The author declares no conflict of interest.

©2015 Montoro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.