eISSN: 2469-2778


Review Article Volume 6 Issue 6
1Dean of Faculty of Medicine, Sabratha University, Libya
2Head of Physiology Department, Faculty of Medicine, Sabratha University, Libya
3Department of Basic Medical Sciences, Inaya Medical College, Saudi Arabia
Correspondence: Azab Elsayed Azab, Head of Physiology Department, Faculty of Medicine, Sabratha University, Sabratha, Libya
Received: November 01, 2018 | Published: December 28, 2018
Citation: Jbireal JM, Azab AE, Elsayed ASI. Disturbance in haematological parameters induced by exposure to electromagnetic fields. Hematol Transfus Int J. 2018;6(6):242-251. DOI: 10.15406/htij.2018.06.00193
Background: The use of mobile phones, wireless, and electrical devices has gradually increased throughout the last century, and scientists have suggested that electromagnetic fields (EMFs) generated by such devices may have harmful effects on living creatures. Many studies revealed that the EMFs might produce a variety of adverse effects on human health as headaches, chronic fatigue, heart problems, stress, nausea, chest pain, and also some bad effects on central nervous, endocrine and immune systems. Exposure to EMF result in deterioration of RBCs function and metabolic activity, it was expected that, the increase of toxicity in specific organs was a result of the RBCs functional failure. The mechanisms by which the electromagnetic fields cause their bad effects may be by causing deterioration in cellular large molecules, imbalance in ionic equilibrium and generation of reactive oxygen species (ROS). These reactive oxygen species can damage cellular components such as proteins, lipids and DNA. Measurements of blood parameters are of the most important diagnostic methods by which we can determine the health status of human and animals for certain diseases as anemia, leukemia and also detect the presence of the inflammations.
Objectives: This study aimed to present an overview on the previous works from 1997 to 2018 on the varying effects of electromagnetic fields on haematological data in human and different species of experimental models by using different frequencies, intensities, and different sources of electromagnetic fields for different periods. The hematological parameters are fluctuating across the exposure period to the EMFs suggesting the possible induction of hazardous biological effects during the exposure to magnetic field.
Conclusion: It can be concluded that exposure of human and experimental animals to EMFs cause harmful effects on blood cells. These effects were disturbance in haematological parameters depending on species, the sources of EMFs, frequencies, intensities and duration of exposure.
Keywords: haematological changes, RBCs, blood smear, blood indices, WBCs, blood platelets, EMF
The use of electrical devices has gradually increased throughout the last century, and scientists have suggested that electromagnetic fields (EMF) generated by such devices may have harmful effects on living creatures.1,2 Since their invention, the telephone has seen much technological advancement up to mobile phones. There are touchtone phones, wireless hand-sets, car-phones, and most recently the cell phone and smart phones have all emerged which are the most widely used recently.3,4 The progress of science will provide the world with new electromagnetic fields emitting technologies and subsequently with new problems. Cell phones have become a vital part of everyday life. It creates an electromagnetic fields around them when in use, thus increasing the electromagnetic contamination.5–7
Human made Electromagnetic sources are kettles microwaves, refrigerators, washing machines, vacuum cleaners, hair dryers, printers, cellular phones, cables that carry electrical currents, television and computers, electrical home gadgets, radio and television base stations, mobile phone base stations and phone equipment, home wiring airport, and transformers.8
Mobile phones, wireless, and electrical devices are the main sources of electromagnetic fields (EMFs). Mobile phones are used in position very close to the human body and require a large number of base station antennas. The resulting health issues have repeatedly been raised by public and scientists.9,10
Innovations in cell phones may be associated with detrimental effects on various organs, systems and their functions.3,4 EMFs might produce a variety of adverse in vivo effects such as chronic fatigue, headaches, cataracts, heart problems, stress, nausea, chest pain, forgetfulness, influence the learning and disturbances in memory, immune systems, and sleep. It has been implicated in adversely affecting multiple facets of human health such as brain, lung and breast tumors, leukemia, genotoxicity, and reproduction anomalies, infertility, increased risk of abortion, birth defects, childhood morbidity, depression, neurodegenerative disease, amyotrophic lateral sclerosis, and Alzheimer’s disease.8 Previous studies showed that an association between elevated EMFs exposure and mortality of employer in electric utility industry jobs from arrhythmia-related causes and acute myocardial infarction influence heart rate variability by changing autonomic balance.2 Exposure to EMFs induces heart palpitations, pain in the chest area, and an irregular heartbeat. Also, exposure to EMFs causes a decreases in total antioxidant capacity and plasma calcium level. Measurements of blood parameters are most important means by which to determine the health status of experimental animals.11,12 These measurements are diagnostic for certain diseases such as anemia, leukemia and detect the presence of the inflammation.12,13 Researchers have shown that microwave radiation from mobile phone causes harmful effects on blood cells in human body. There is increase in red blood cells (RBCs) count, decrease in white blood cell (WBCs) count and lymphocytes count after prolonged exposure to microwave radiation.10 Rats exposed to EMF show increases in blood pressure, the whole heart and left ventricular weights.2 Movement of hemoglobin in blood vessels is accelerated due to presence of ferric ions.14,15
EMFs have various chemical effects, including causing deterioration in large molecules in cells and imbalance in ionic equilibrium. Despite being essential for life, oxygen molecules can lead to the generation of hazardous by-products known as reactive oxygen species (ROS) during biological reactions. These ROS can damage cellular components such as lipids, proteins, and DNA. Antioxidant defense systems exist in order to keep free radical formation under control and to prevent their harmful effects on the biological system. Free radical formation can take place in various ways, including ultraviolet light, immunological reactions, radiation, stress, smoking, and biochemical redox reactions. Oxidative stress (OS) occurs if the antioxidant defense system is unable to prevent the harmful effects of free radicals. Exposure to EMF is known to increase free radical concentrations and trace ability and can affect the radical couple recombination.16 Electromagnetic waves have been shown to exert their effects on biological systems through generation or increase in ROS. ROS, as a medium, contributes to numerous biological impacts including DNA damage and mutation induction.17–19 The increase in OS in hematopoietic centers has been reported due to use of mobile phone.18–20
This study aimed to present an overview on the previous works from 1997 to 2018 on the varying effects of electromagnetic fields on haematological data in human and different species of experimental models by using different frequencies, intensities, and different sources of electromagnetic fields for different periods.
Effect of EMF on red blood corpuscles and its indices
Table 1 shows the effect of exposure of experimental animals and human to EMFs from different sources, frequency, and intensity on RBCs count, Hb content, Hct value, MCV, MCH, and MCHc. Researchers have shown that the electromagnetic fields created by mobile phones and many electronic devices causes harmful effects on red blood cells and its indices in human and experimental animals.
Species |
Exposure frequency |
Exposure parameters |
Exposure |
Results |
References |
Mice |
900MHz |
- Incident power |
30-min periods per day for up to 18 months |
A significant decreases in RBCs count, Hb content, and Hct value, MCV and MCH |
|
Male Human (Welders from 20 to 40 years of age) |
ELF-EMF |
10μT– 2 T |
3–4 h /day/week & the subjects have been welding for at least 10 years. |
A significant increase Hct value. |
|
Wistar rats |
100Hz |
- |
10 min/day for 5, 10 and 20days. |
A significant increase in RBCs count, Hb content, Hct value, and MCH. |
|
Male Wistar rats |
SMF |
128mT |
1h/day for 30 days |
A significant increases in RBCs count and Hb content. |
|
Male Wistar rats |
SMF |
128mT |
1h/day for 30 days |
A significant increases in Hb, RBCs count. |
|
Sprague Dawley rats |
2450MHz |
1&10Mw/Cm-2 |
exposed for one year |
A significant increase in RBCs count, Hb content, and Hct value. |
|
Male mice |
650MHz |
- |
5 months |
A significant decrease in RBCs count |
|
Female Swiss albino mice |
50 Hz |
2mT |
4h /day for 30 days |
A significant increases in RBCs count, Hb content, and Hct value |
|
Female Wistar rats |
ELF-EMF |
0.97mT |
3h/day for 50 and 100 days |
A significant decrease in Hb content. |
|
Albino rats |
900 MHz |
(1.4-4.7)mW/Cm2 |
2 weeks |
A significant decreases in RBCs count, Hb content, Hct value, MCV, and MCH. |
|
Mice |
2.45GHz |
0.02564mW/cm2 |
2 hrs/day for 30 days |
Alteration in blood pictures |
|
Male albino rats |
SMF |
2mT |
60 minutes, 3 days per week for two weeks |
-After one week, an increase of RBCs count, Hb content, and Hct value. A significant decrease in blood iron. |
|
Swiss albino mice |
0.9 GHz and 1.8 GHz |
- Field strength = 0.6 x10-3 mW/cm2 |
7 h/day for 12 weeks. |
-A significant increase in RBCs count and Hct value, and a significant decrease in MCHC. |
|
Male white Balb/c mice |
Mobile phone (Alcatel& Nokia) |
The mobile phone Alcatel: 0.49 W/kg. |
- Short term experiment: 15,30, 45 & 60 min / day for 2 weeks |
- A decline in RBCs count, Hb content, and Hct value. |
|
Male albino rats |
EMF |
202μT |
|
A significant increase in RBCs, and Hb as well as decrease in red blood cell indices values of MCV, MCH and MCHC |
|
Swiss albino mice |
10 GHz |
0.25mW/cm2 SAR=0.1790 W/Kg |
2 h/day for 30 day. |
An increase in MCV, and a significant decrease in RBCs count, Hb, Hct, MCH, and MCHC |
|
Males mice |
1200 MHz |
- |
6 h/day for 45 days. |
-A significant increase in RBcs count. |
|
Male albino rats |
900MHz |
1.4 mW/cm |
2hrs/day, 3 times/week for 2 months |
A reduction in RBCs, Hb, Hct, MCV, MCH and MCHC. |
|
Human |
Microwave radiation from mobile phone (Dual band EGSM 900/1800 MHZ) |
- |
Blood sample was exposed to RF-EMF for one hour in talk mode inside the wooden box. |
A significant increase in RBCs count. |
|
Male albino rats |
900MHz |
A peak power of about 60W, power density of 0.05mW/cm2 |
24h/day for 8 Weeks |
a significant reduction in RBCs counts, and Hb concentration, meanwhile reticulocyte count was elevated |
|
Male albino rats |
900MHz & 1.3GHz |
- |
1, 2, 5h/day for 28days |
A significant increase in RBcs count, Hb and MCH |
|
Adult female rat |
SMF & mobile |
- |
30min/day for 45days |
There were significant (p≤0.05) decrease in RBCs count when exposed to electromagnetic field and mobile radiation. |
Table 1 The effect of EMFs on RBCs Count and its indices in human and animals.
CW, continuous wave; ELF-EMF, Extremely low frequency electromagnetic fields; GSM, Global System for Mobile Communication; RF-EMF, Radiofrequency electromagnetic fields; SAR, Specific absorption rates; SMF, Static magnetic field
Effects of EMFs on blood smears
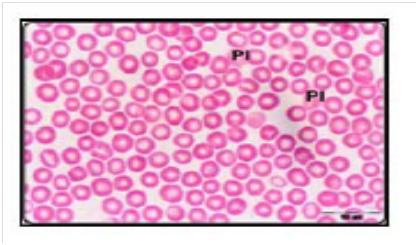
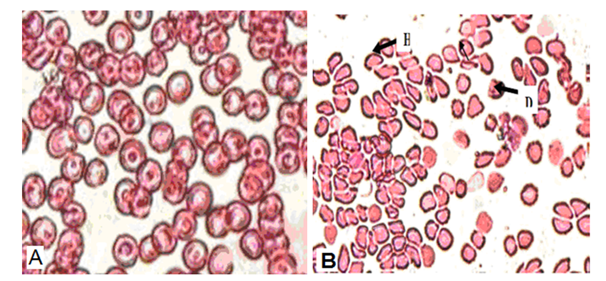
Alghamdi and El-Ghazaly,12 investigate the effects of electromagnetic fields on blood smears of male white Balb/c mice. In this study two experiments were used as short-term and long-term experiments. Short-term experiments were carried out in 80 male white Balb/c mice exposed to two types of mobile phone for a period time up to 60min a day for 2 weeks. Long-term experiments were carried out in 30 male white Balb/c mice exposed to two types of mobile phone for a period time up to 90min a day for a month, two months, three months. Authors found that when exposed male white Balb/c mice to both devices (Alcatel, Nokia) for 15 minutes, the red blood cells began to take a role of formation (Figure 1A-B) which may be due to an increase in the viscosity of the blood and the ability of the cells adhesion. Increased duration of exposure to 30-minute to both devices revealed disparities in the sizes of red blood cells with the appearance of a large proportion of cells with pale colors (Figure 1C) which may be due to lack of hemoglobin. In the group exposed for 45 minutes showed clearly the influence of mobile phones on examining blood smears which observed a proportion variation in the cell sizes with the emergence of forms of abnormal forms, including pellets cleft with many areas empty of red blood cells (Figure 1D). With increasing in of exposure duration to 60minutes, red blood cells appeared completely different from the natural form and took the forms resembling the eye and tear that appear in the case of anemia (Figure 1E). After three months of exposure to both types of mobile phone showed pathological changes in red blood cells where gripped the outer membrane of the red corpuscle changes and become serrated (Figure 1F).

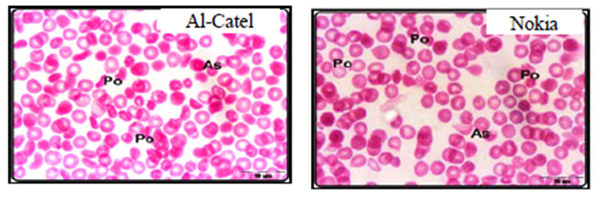

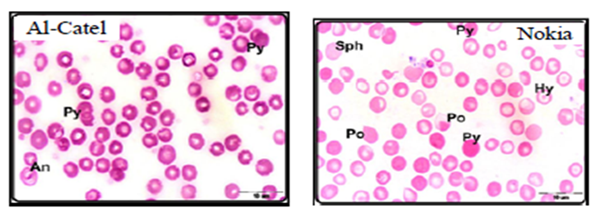
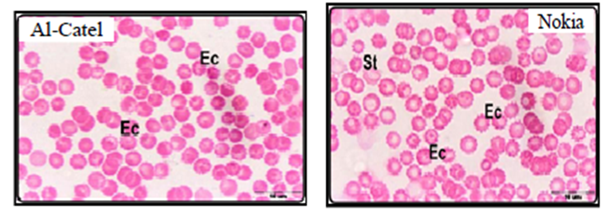
The study of Rifat et al.,21 showed that microscopic examination of blood smears of Swiss albino mice exposed to10GHz microwave (MW) with the power density of 0.25mW/cm2 with average whole body specific absorption rate 0.1790W/kg/day for 2h/day for 30 day caused a modifications in the shape and size of the erythrocytes representing morphologic abnormality as compared with unexposed mice to MW (Figure 2). Poikilocytosis, spherocytosis, hemolysed and distorted erythrocytes seen in MW exposed mice may be due to the formation of active loci on the red blood cell membrane as a result of interaction with free radicals thereby resulting in the alteration of shape of erythrocytes.21
Effects of EMFs on leukocytes
The effects of EMFs on WBCs count, lymphocytes %, neutrophils %, eosinophils %, basophils %, and monocytes % in human and experimental animals are shown in Table 2.
Animal species |
Exposure frequency |
Exposure parameters |
Exposure duration |
Results |
References |
Male Human (Welders from 20 to 40 years of age) |
- ELF-EMF |
10μT– 2 T |
3–4h/ day/week |
-Insignificant changes in WBCs count, neutrophils, lymphocytes, and eosinophils |
|
Wistar rats |
100Hz |
- |
10min/day for 5, 10 and 20 days. |
The total leucocytes number fluctuated: decrease at 5 and 20 days and increase at 10 days of treatment. |
|
Male Wistar rats |
SMF |
128mT |
1h/day for 30days |
A significant increase in WBCs count. |
|
Male Wistar rats |
SMF |
128mT |
1h/day for 30days |
A significant increase in WBCs count. |
|
Female Wistar rats |
ELF-EMF |
0.97mT |
3h/day for 50 and 100 days |
A significant decrease in Eosinophils in rats that were exposed to EMF for 50 days. |
|
Female Swiss albino mice |
50 Hz |
2 mT (=20 Gauss) |
(4h /day) for 30days |
-Leukocytosis with neutrophilia, lymphocytosis, and monocytosis |
|
Albino rats |
900 MHz |
(1.4-4.7) mW/Cm2 |
2weeks |
-A significant increase in WBCs count and lymphocytes % |
|
Male albino rats |
SMF |
2mT |
60min/day (3 days per week) for two weeks |
A significant increase in WBCs count . |
|
Male white Balb/c mice |
Mobile phone (Alcatel and Nokia) |
The mobile phone Alcatel:0.49W/kg. |
-Short term experiment: 15,30, 45 & 60min a day for 2 weeks |
A significant increase in WBCs and lymphocytes count. |
|
Swiss albino mice |
0.9 GHz & 1.8 GHz |
0.6 x 10-3mW/cm2 |
7 h/day for 12 weeks. |
A significant decrease in WBCs count. |
|
Male albino rats |
the electromagnetic field |
202μT |
|
A significant increase in WBCs count. |
|
Swiss albino mice |
10 GHz |
0.25mW/cm2 (0.1790 W/Kg) |
2 h/day for 30 day. |
A significant increase in WBCs count, and lymphocytes %, and a significant decrease in monocytes %. |
|
Males mice |
1200 MHz |
- |
6 h/day for 45 days. |
-A significant increase in WBCs count, lymphocytes, monocytes and acidophils. |
|
Male albino rats |
900MHz |
1.4mW/cm |
2hrs/day, 3 times/week for 2 months |
-A significant increase in WBCs count, lymphocytes % , and neutrophil %. |
|
Human |
Microwave radiation from mobile phone |
- |
Prolonged exposure |
-A significant decrease in WBCs and lymphocytes count. |
|
Male albino rats |
900MHz |
A peak power of about 60W, power density of 0.05mW/cm2 |
24h/day for 8 Weeks |
- Leukocyte counts was not affected by exposure |
|
Male albino rats |
900MHz & 1.3GHz |
- |
1, 2, 5h/day for 28 days |
-Lymphocytes are fluctuating across the exposure period . |
|
Adult female rat |
SMF and mobile |
- |
30 min/day for 45 days |
A significant ( p≤0.05) increase in WBC when were exposed to electromagnetic field. While there were a significant (p≤0.05) decrease in WBC when female rats were exposure to mobile phone radiation. |
Table 2 The effect of EMFs on WBCs Count and Differential Count in human and animals
CW, continuous wave; ELF-EMF, Extremely low frequency electromagnetic fields; GSM, Global System for Mobile Communication; RF-EMF, Radiofrequency electromagnetic fields; SAR, Specific absorption rates; SMF, Static magnetic field
Effects of EMFs on blood platelets
Table 3 shows the effect of EMFs on blood platelets count in human and experimental animals.
Animal species |
Exposure frequency |
Exposure parameters |
Exposure duration |
Results |
References |
Male Human (Welders from 20 to 40 years of age) |
|
10μT– 2 T |
3–4 h per day. |
Insignificant changes in platelets count. |
|
Male Wistar rats |
SMF |
128mT |
1h/day for 30 days |
An increase in platelet count. |
|
Male Wistar rats |
SMF |
128mT |
1h/day for 30 days |
An increase in platelet count. |
|
Female Wistar rats |
ELF-EMF |
0.97mT |
3h/day for 50 and 100 days |
Non significant changes in platelet count and PDW levels. |
|
Female Swiss albino mice |
50 Hz |
2mT (=20 Gauss) |
(4h/day) for 30 days |
An increase in the blood platelets count. |
|
Albino rats |
900 MHz |
(1.4-4.7) mW/Cm2 |
2 weeks |
- A significant increase in the blood platelets count |
|
Male albino rats |
SMF |
2mT |
60minutes, 3 days per week for two weeks |
A significant increase in the blood platelets count. |
|
Male white Balb/c mice |
Exposed mice to two types of mobile phone (Alcatel and Nokia) separately |
The mobile phone Alcatel: 0.49 W/kg. |
-Short term experiment: 15,30, 45 & 60 min/day for 2 weeks |
A decline in the platelets count. |
|
Male albino rats |
the electromagnetic field |
202μT |
|
A significant increase in platelet count |
|
Male albino rats |
900MHz |
1.4 mW/cm2 |
2hrs/day, 3 times /week for 2 months |
An increase in the platelets count |
|
Male albino rats |
electromagnetic field, emitted from a cellular tower for mobile phone 900MHz |
A peak power of about 60W, power density of 0.05mW/ cm2 |
24h/day for 8 Weeks |
- Blood platelets count was not affected by exposure. |
Table 3 The effect of EMFs on Blood platelets Count in human and animals
CW, continuous wave; ELF-EMF, extremely low frequency electromagnetic fields; GSM, global system for mobile communication; RF-EMF, radiofrequency electromagnetic fields; SAR, specific absorption rates; SMF, static magnetic field
EMF had a different influence on RBCs and leucocytes Count. A such an effect can be determined by a selective action of EMF on haematopoietic processes and the cell maturation rhythm and on sanguine reservoir.24 Interpretation of EMR effect on blood and blood forming systems depends to a great degree on the absorption of biological material and thermoregulatory system of the irradiated individual.40,41 Many of previous researches reported that exposure of some experimental models to EMF caused a decrease in RBCs count and its indices.12,21,22,28,37-39 The decrease in RBCs count in animals exposed to EMF can be explained by the effect of EMR on the haematopoietic system which is susceptible to be damaged by radiation and that the high mitotic activity of the bone marrow make it the most vulnerable organ system to the effect of radiation.38,42,43 This decline is an indication of different types of anemia (Singh et al. 1995). These findings were further confirmed histopathologically in the spleen which showed splenic sinusoids with hemorrhagic or/and hemolysed blood and an increase of hemosiderin granules.33 This may arise under the influence of exposure to radiation, increased temperature and body resistance.12 The depletion in the values of hematological parameters (RBCs, Hb, Hct, MCV, MCH and MCHC) following EMF radiation exposure may be attributed to direct damage caused by radiation and due to overproduction of ROS by microwave radiation interaction21,37,44 and inhibition by free radicals produced by MW interactions.21 The effects of free radicals on the red blood cell membrane and cytoskeleton may contribute to the leak of hemoglobin out of the cells.36,37,45–48 The hemolysis of the red blood cells reflects the loss of integrity of the cells which can lead to the liberation of intracellular hemoglobin.37,47 Haemolytic disorders may be caused by many factors including physical agents.38,49 The haemolytic anaemia results primarily from increased red cell destruction38,50 which stimulates erythropoiesis. The evaluation of suspected haemolytic states should include measures of increased red cell destruction and parameters of accelerated erythropoiesis which is measured by the reticulocyte count. These results could be explained on the base of EMR exposure may be responsible for the changes of blood and glutathione enhancer can provide protection against the effect of EMR of MBS.51,52 Radiation was reported to cause oxidation of the sulphydryl groups and induce conformational changes of membrane proteins.37,53 The hypothesis of an action of SMF on the geometrical conformation of haemoglobin was reinforced by the fact that SMF induced a prominent effect on the haemoglobin structure as previously demonstrated by Amara et al.,26 and Zaghloul.33 Exposure to moderate and strong SMF induced change in the absorption spectra and conductivity measurements of hemoglobin molecules, globin unfolding i.e. a sign of molecular destabilization. This reflects the function of haemoglobin which would be converted from oxyhaemoglobin to non functional met haemoglobin with decreasing oxygen affinity.33,54 The SMF may cause cardiovascular stress accompanied with a slow development of mild cardiac decompensation during the exposure period, hence developing heart failure with subsequent passive congestion and stagnant hypoxia.33,55 Thus, the body adapts to the higher oxygen needs, more fluid would be in the blood. In turn, measured haemoglobin in such cases would be apparently low. This is because it was diluted out by a larger plasma volume. Moreover, the red blood cells looked normal on the blood smear although haemoglobin, serum iron and haematocrit values were significantly decreased, hence resulted in anemia. Usually, this type of anemia is mild and appears like the pseudo-anemia caused by sports. In this respect, regular physical activity, especially extensive running and exercises increase iron loss causing mild iron deficiency.33,56
Packed cells volume was a significant decrease as compared with the control group, which can be explained as due to a significant decrease in Hb, this would lead to decrease in the size of RBCs and hence a final decrease in PCV.36
The decrease in blood iron could be attributed to the interaction between heme (iron) and SMF where the magnetic field penetrates the body and acts on ions in all organs, altering the cell membrane potential and distribution of ions.33,57 It is well documented that transferrin controlled transit of iron since intestinal enterocytes increase medullar erythroblasts and allowed recovery of iron after destruction of erythrocytes by macrophagic system.33,58
On the other hand, other researches reported that a significant increase in RBCs count, and most of RBCs indices were recorded after exposure of different species of animals to EMFs.4,10,24–27,29,33–36 Thus, indicating that long time exposure might pose detrimental effects to blood components and their functions.4
Exposure to EMF result in deterioration of RBCs function and metabolic activity, it was expected that, the increase of toxicity in specific organs was a result of the RBCs functional failure. Therefore, changes in antioxidants may be due to the deterioration in cellular membrane properties in the liver. In addition to increase toxicity in different organs.27,59 Magnetic field exposure has been reported to increase the immature and mature erythrocytes in mice.15,60 These changes suggesting the hypoxia-like status resulting probably from the oxygen-binding impairment of Hb which in turn associates with increased oxidative stress.26,29,36,61–63
Hemoglobin content record an increase values according to increasing of MCH values, indicating a better level of respiratory performances of RBCs count as a result of EMF action. The increasing of hemoglobin values takes places especially by an increase of MCH and less by an increase of RBCs count. This effect assume a hemoglobin synthesis stimulation by EMF. MCV recorded low values, possible owing to an effect of EMF on membrane permeability with a lost of the cellular water and a cellular volume reducing. This effect is accompanied by the MCH increase with preserve of cellular respiratory capacity.24
Many of previous studies showed that exposure of some experimental models to EMF caused a significant increase in WBCs count and some differential WBC counts over time. 12,21,25,26,29,31,33,35,36,39,64 Elevated WBC count might reflect inflammation.29 A significant increase in white blood cells count were accompanied by enlargement of the white pulp masses was detected one day after the end of exposure to SMF. It is clear that a splenic white pulp represents an active site of lymphocytic cell proliferation.33 The significant increase in total WBCs count comes from the significant high increase in lymphocytes which is due to the harmful action of electromagnetic waves exposure that stimulates the haemopoietic system to release more lymphocytes causing an increase in their number in the blood stream.21,31,36,37
Abdel Aziz et al.,31 reported that a significant increase in lymphocytes in cases of macrocytic anemia, which arise under the influence of exposure to radiation, increased temperature, and increased resistance to the body’s immune system. The increase in the lymphocytes % may be associated with lymphatic leukemia or inflammation of the lymph gland which will appear as a result of constant exposure to electromagnetic waves. 65–67 These results were confirmed by lymphoid depletion in some splenic white pulps and the others became hyperplastic.29
Exposure to EMFs caused a significant decrease in neutrophils,36,68 which might be due to the absolute increase in lymphocytes (lymphocytosis).36
On the other hand, other researchers reprted that exposure of experimental animals to EMFs caused a significant decrease in WBCs count.4,10,15,34,69–71
Bonhomme- Faivre et al.,69 reported a decline in lymphocytes, monocytes and eosinophils in mice depending on the duration of exposure to magnetic field. Also, it has been reported that chronic exposure to a 0.2-6.6-μT magnetic field can lead to decreased total lymphocytes in humans and mice.15,72
Most of the previous studies reported that exposure of experimental animals to EMFs resulted in a significant increase in blood platelets count.25,26,29,31,33,35,37 These results were confirmed by proliferation of megakaryocytes, precursor cells of blood platelets.29,33,73 The increase in megakaryocytes may lead to increased blood platelets which were observed.37
The variation in the haematological parameters can be attributed to the differences in the frequency of EMF used and/ or duration and time of exposure, the mitotic rate of the exposed cell population and the differences in animal species used.38,74
It can be concluded that exposure of human and experimental animals to EMFs has been causes harmful effects on blood cells. These effects were disturbance in haematological parameters depending on species, the sources of EMFs, frequencies, intensities and duration of exposure. The EMFs exposure guidelines also need to be updated or reconsidered as new scientific information on EMF and the haematological parameters is produced. However, additional studies might increase our understanding of the sensitivity of haematopieses to EMFs.
None.
Author declares that there is no conflicts of interest.

©2018 Jbireal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.