eISSN: 2576-4497


Research Article Volume 3 Issue 3
1Department of Otorhinolaryngology, Federal University of São Paulo, Brazil
2Adjunct professor, Department of Pediatrics, Federal University of São Paulo, Brazil
3Titular professor, Department of Pediatrics, Federal University of São Paulo, Brazil
Correspondence: Adriana Garófolo, Rua Manoel da Nóbrega, 1088, ap. 131, Paraíso, São Paulo (SP), Brasil, Tel (+55 11) 9712-8293
Received: April 18, 2019 | Published: June 4, 2019
Citation: Garófolo A, Petrilli AS, Ancona-Lopez F. Outcomes of an enteral feeding protocol in pediatric cancer patients: a non-randomized clinical trial. Hos Pal Med Int Jnl. 2019;3(3):103-111. DOI: 10.15406/hpmij.2019.03.00161
Context and objective: Tumor therapy has adverse effects on nutritional status among cancer patients, leading to nutritional deficits. The aim was to study the refusal and nutritional impact of the tube feeding in pediatric cancer patients. Clinical trial at Pediatric Oncology Institute – Federal University of São Paulo.
Design and setting: A clinical trial, carried out from January 2002 to January 2004 at a Public University in São Paulo.
Methods: Patients over one year old followed during anticancer therapy were included. They received commercially prepared oral supplementation (OS) and were followed weekly, with revaluations in weeks 3, 8 and 12. When inadequate outcomes were observed, tube feeding (TF) with the same supplement was indicated.
Results: One hundred and forty-one patients were invited to participate in the study; 117 agreed to follow up, 47 had TF indication. Five out of 47 were excluded; 23/42 accepted to use TF, but 19 refused and were followed by OS. The length of stay with TF was 5.78±4.99 weeks. Patients receiving TF had higher nutritional intake than those with OS and their intake was higher during TF than before TF. Comparing the week zero with the last week with TF they demonstrated significant improvement in triceps skinfold thickness (p<0.001), mid-upper arm circumference (p<0.001) and arm muscle circumference (p<0.001), showing that the longer the length of stay with the TF, the better the nutritional evolution (p<0.01).
Conclusion: These results suggest that refusal of the enteral TF is high, but to use TF demonstrated improvement in nutritional outcomes, particularly in the longer term.
Keywords: enteral nutrition, nutritional status, neoplasms, child, adolescent
Cancer treatment is a catabolic process that depletes the host’s stores, thereby leading to metabolic changes and malnutrition.1 The importance of nutrition is based on the fact that organ functions are more adequately maintained if the nutritional status is preserved.2–4 Although some adaptive mechanisms might preserve life in malnourished patients, malnourished children with cancer have their organ systems damaged also by toxins from antineoplastic drugs. The ability of the liver and gastrointestinal tract to absorb and metabolize drugs and nutrients may be impaired under conditions of malnutrition.3,4 Both cancer therapy and malnutrition lead to immune function damage, thus increasing the risk of infections and morbidity-mortality.5–7 Gastrointestinal side effects from the treatment, like severe nausea (grade 3 or 4) and vomiting, mucositis (sore mouth or throat), diarrhoea and constipation, are the main nutritional difficulties.
Despite evidence that malnutrition relates to complications and disease relapses8,9 definite conclusions have been reached concerning the advantages of enteral nutrition in such patients.10–12 Prospective clinical trials have been performed more frequently among adults receiving parenteral nutrition.13–18 Although the enteral route has many theoretical advantages, studies have failed to provide definite conclusions.19–23 However, a few studies have been conducted with this aim over the past few years. Overall, they have shown improvements in nutritional status without worsening of gastrointestinal symptoms from chemotherapy.24,25
The aim was to study the refusal and impact of the tube feeding on nutritional status in pediatric cancer patients, through applying a protocol that was based in an algorithm for nutritional support decision-making, already published previously (reference cited in supplementary document). The mean hypothesis was that supplement intake by TF improves nutritional status in pediatric cancer patients.
This clinical trial was carried out at Pediatric Oncology Institute, São Paulo Federal University (UNIFESP), in Brazil, among patients diagnosed with cancer. The patients were followed during anticancer therapy from January 2002 to January 2004. The inclusion criteria were: (1) performing chemotherapy protocol; (2) over one year of age, and (3) presence of malnutrition. Weight for height (W/H) Z-scores from <-1.0 to -2.0 among children26 and body mass index (BMI) from greater than or equal to the fifth percentile to greater than the fifteenth percentile among adolescents27 were considered mild malnutrition; W/H Z-scores <-2.0 in children and less than the fifth percentile of BMI in adolescents as severe, respectively.28 In adults, the World Health Organization (WHO)29 cut-off values were applied: <18.5 for mild and <17 for severe malnutrition. The exclusion criteria were (1) corticosteroids or hormonal therapy; (2) swallowing abnormalities, (3) early or exclusive parenteral nutrition support, (4) previous or current use of tube feeding support, (5) palliative care and (6) presence of non-cancer-related diseases.
Study design
The study was a part of a protocol that applied an algorithm for nutritional support decision. This step was a controlled non-randomized clinical trial. This analysis followed patients prospectively with commercially prepared oral supplementation (OS) and nasoenteral tube feeding (TF) regimens in an outpatient setting. In cases of hospitalization, the follow-up was continued into the hospital, in accordance with the same schedule. For the analysis, the patients were evaluated for three weeks of each one period: OS before the TF indication (period 1); on TF (period 2); and after TF discontinuation (period 3), when the patients were maintained on OS. Another analysis was carried out with the intention of evaluating the nutritional outcome after monitoring the total period of use of the TF.
An ideal response of severely malnourished patient was defined by means increase ≥0.3 standard deviations (SD) of the W/H Z-score or ≥3% of the BMI, and a negative response was considered when mildly malnourished individuals became severely malnourished. When an apparent tumor masses that might overestimate the weight were detected, triceps skin fold thickness (TSFT) and mid-upper arm circumference (MUAC) methods were used to recommend TF support by the following criterion: TSFT and MUAC were below the 5th percentile. Grades three and four gastrointestinal toxicity, tumors with a risk of bleeding, and platelet count less than 30,000 cells/mm3 were the clinical conditions that could delay nutritional support after its indication.
Oral and tube feeding regimen
The oral and tube supplement offered was a polymeric powder standard formulation (52% carbohydrates, 12% proteins and 36% lipids, taurine, L-carnitine, vitamins and minerals, presenting 1.0 kcal/ml). Patients were followed weekly for supplementation advice and nutritional counseling, and were encouraged to eat according to their individual custom. The patients were encouraged to reach 100% if they could tolerate and accept it.30–32
The daily energy intake offered was in accordance with the recommendations for pediatric and juvenile cancer patients, by means appropriated equations.33–36 The total daily energy requirements were adjusted in accordance with the protocol: it started with 50% of the recommended energy intake, increased to 75% over three consecutive days and could reach 100% in five days, depending on gastrointestinal tolerance. The amount could be incremented if the nutritional status had not improved one week after the last increase. The recovery factor used to correct for weight loss was determined by dividing the patient’s ideal weight (W/H or BMI) by the actual weight.
Nausea and vomiting were minimized by means of a standardized antiemetic regimen for anticancer therapy (Ondansetron; 0.15 mg/kg, 3 times a day). When patients suffered consistent vomiting above a certain amount, the volume of feeding was reduced to the previously tolerated level, and it was increased again when the tolerance improved.
TF was administered via a small-bore silicone duodenal feeding tube (“Nutricia Flocare, United Kingdom”) that was inserted into the stomach (nasoenteral tube feeding). During hospitalization, tube feeding was administered by continuous or intermittent infusion over a 24-hour period, depending on the clinical conditions. At home, overnight feeding was avoided because infusion pumps were not available. Oral feeding was permitted ad libitum. Enteral supplement intake was recorded weekly, based on what the patients actually received during the week, relying on parents’ memories. This method is based on seven-day recall, which is used to assess food intake.37
Nutritional assessment
The BMI percentiles and W/H Z-scores were classified in accordance with WHO (< 5th and <-2,00 SD) (28,29). Mild malnutrition among adolescents was classified based on data published by Lusky (<15th to 5th) (27), and for children a cut-off was proposed, with the aim of preventing severe malnutrition (< -1,00 to -2,00 SD).26
TSFT, MUAC and arm muscle circumference (AMC) were the indicators used for follow-up and outcome analysis by scientific caliper (Harpenden model; Cescorf). These variables were measured an interpreted in accordance with the guidelines and percentile charts.38
Nutritional outcomes analysis
The assessments were performed at the time of inclusion and weekly thereafter. The data analyzed were those from week zero and week three of oral supplementation; week zero, week three and the last week of TF; and week zero, week three and the last week of oral supplement (in patients that refused TF).
For the patients undergoing TF, an evaluation was also made after they stopped TF and went into OS, following also for three weeks of that period. The means of supplement intake was analyzed to compare the group that used the TF before and after its introduction and to compared the two groups (TF versus OS) in period 2.
Clinical and gastrointestinal toxicity assessment
Gastrointestinal toxicity grades were applied to evaluate nausea, vomiting and diarrhea.39 Episodes of hospitalization and leukocyte, platelet and haemoglobin concentrations were measured in order to evaluate the clinical outcomes from tube feeding and oral supplementation.
Ethics committee
The nutritional support protocol from which this analysis was derived was approved by the Institutional Review Board/Ethics Committee of Unifesp-EPM protocol number: 1097/02 (17/03/2003). Informed consent was obtained from parents, guardians or patients, after the study protocol was explained to them.
Statistical analysis
The statistical software used was the Number Cruncher Statistical Software (2000, NCSS version I, Hintze, Kaysville, Utah, United States). The chi-squared test was used to compare differences between TF and OS, in relation to categorical data: diagnosis (solid or hematological tumors), age (<10 years or ≥10 years), gender, educational level and weeks of supplementation (≥or <8 weeks). This test was also applied to compare hospitalization (TF versus OS) during periods 1 and 2. The paired Student t or Wilcoxon test were used to compare the evolution between period 1 and 2 and the two-sample Student t or Mann-Whitney test was performed to calculate differences between the TF and OS groups, depending on curve distribution and variance. The same tests were used to compare the nutritional status between TF and OS patients in week zero of TF indication.
Simple linear regression analysis was used to study associations of gastrointestinal toxicity and clinical indicators with nutritional outcome variables from week zero to the last week (changes in the percentages of the ideal TSFT, MUAC and AMC), among TF patients. Linear regression analysis was also performed to study associations between supplement intake by TF or OS patients and gastrointestinal symptoms in period 2, and between amount of supplement intake and gastrointestinal toxicity among TF patients. This analysis also studied associations between age (in years) and length of TF stay (in weeks), and between categorical age (children and adolescents) and gastrointestinal toxicity.
Multiple regression analysis was performed to study associations between duration of TF (in weeks) and percentage of supplement achieved with TF, and between nutritional indices from week zero to the last week. The educational level of the primary caregiver, three specific diagnostic categories (hematological, solid and bone tumors) and age category were investigated as potential confounders.
The sample size of 27 patients for the TF regimen was calculated by taking the main effect of this study to be the nutritional outcome between periods 1 and 2. An effect of 20% was calculated, using α=0.05, β=0.20 and a test power of 80%.
For the regression analyses, the number of variables was not greater than 15% of the number of subjects.
All the statistical tests were two-tailed. P-values below 5% or 0.05 were taken to be significant.
Overall descriptive analyses
One hundred and forty-one patients were invited to participate in the study; 117 agreed to follow up; 47 had indication for TF (40.5%). Figure 1 shows a flowchart with a detailed description of the follow-up of the patients after the indication of the TF. After exclusion criterias, a total of 42 individuals were followed for the nutritional outcomes: 23 in the TF group and 19 in the OS group.
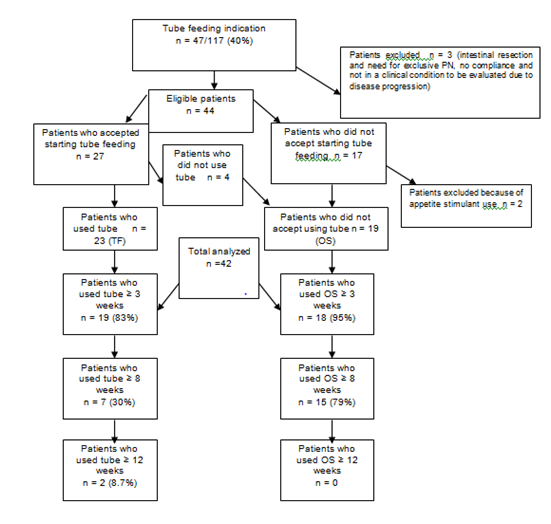
Figure 1Descriptive analysis of patients flow after tube feeding indication.
PN, parenteral nutrition; OS, commercially prepared oral supplementation; TF, tube feeding; SM, severe malnutrition; MM, mild malnutrition; OS, oral supplementation
The main causes of non-acceptance of the TF regimen were patient refusal or parent rejection, with preference for the use other strategies. The analysis of the 23 patients who received TF demonstrated that 18 of them had the tube inserted within the same week that the TF indication was done, but five were placed later. The primary causes for this delay were low platelet count (n=2), grade 3 mucositis (n=1), non-attendance at the nutritional consultation (n=1) and septic shock (n=1).
The age range in the total sample was 1 to 21 years old; there were 10 children (<10 years) and 32 adolescents and young adults (≥10 years). The comparison between patients with TF and OS, according to gender, age, educational level and diagnosis, did not demonstrate any statistical differences (Table 1).
Nutritional regimen |
Diagnostics |
n |
Children |
Adolescents |
Male |
Female |
TF |
Acute lymphoblastic leukemia |
1 |
- |
1 |
1 |
- |
Hodgkin’s lymphoma |
1 |
- |
1 |
1 |
- |
|
Bone tumors |
10 |
1 |
9 |
4 |
6 |
|
Brain tumors |
1 |
- |
1 |
1 |
- |
|
Neuroblastoma IV |
3 |
2 |
1 |
2 |
1 |
|
Wilms’ tumor IV |
1 |
1 |
0 |
0 |
1 |
|
Rhabdomyosarcoma |
3 |
1 |
2 |
1 |
2 |
|
Germ cell tumor |
1 |
1 |
0 |
0 |
1 |
|
Others |
2 |
2 |
0 |
2 |
0 |
|
Subtotal |
23 |
8 (35%) |
15 (65%) |
12 (52%) |
11 (48%) |
|
OS |
Acute lymphoblastic leukemia |
2 |
1 |
1 |
1 |
1 |
Hodgkin’s lymphoma |
1 |
- |
1 |
1 |
- |
|
Bone tumors |
7 |
- |
7 |
2 |
5 |
|
Brain tumors |
3 |
- |
3 |
2 |
1 |
|
Wilms’ tumor IV |
1 |
1 |
- |
- |
1 |
|
Rhabdomyosarcoma |
1 |
- |
1 |
1 |
- |
|
Others |
4 |
- |
4 |
3 |
1 |
|
Subtotal |
19 |
2 (10.5%) |
17 (89.5%) |
10 (53%) |
9 (47%) |
|
Total |
42 |
10 (23.8%) |
32 (76.2%) |
22 (52.4%) |
20 (47.6%) |
Table 1 Demographic characteristics of 42 cancer patients
Statistical analysis (chi-squared): p = non-statistically significant differences between OS and TF for age (p=0.08; OR=0.22: 0.04-1.20; 95% CI), gender (p=0.98; OR=1.02: 0.30-3.44; 95% CI) , diagnosis: solid non-hematological versus hematological malignancies (p=0.49; OR=0.51: 0.08-3.41; 95% CI) and educational level (p=0.68; OR=1.69: 0.49-5.85; 95% CI).
TF, tube feeding; OS, commercially-prepared oral supplementation
The mean and median of length of stay with the TF regimen for 23 patients were 5.78 ± 4.99 and 5 (1-23) weeks, respectively. Nineteen (83%) remained on the TF regimen for three weeks or more, seven (30.4%) for eight weeks or more and two (8.7%) for twelve weeks or more.
Comparing OS versus TF, there were borderline differences in adequacy of nutritional indicators in period 2, according to MUAC (73.5% versus 76.9%; p = 0.06) and AMC (77.9% versus 82%; p=0.08), and statistical differences, according to the BMI for adolescents (71.9% versus 77.3%; p=0.02).
Three of the overall patients required a short period of parenteral nutrition during follow-up due to perioperative complications (2) or gastrointestinal toxicity (1); two of them were on a TF regimen and one on OS (mean, 6.5 days).
Twenty-one patients used a nasogastric tube and two of them needed a nasoduodenal/jejunal tube because of stomach tumor compression and mechanical ventilation. Formula modification (polymeric to oligomeric) was needed for three patients due to diarrhea from abdominal radiation (n=1), diarrhoea from chemotherapy (n=1) and diarrhoea from intestinal resection (n=1). The causes of tube extubation were vomiting in 17 cases (63%), four achieve nutritional adequacy (15%), four had accidental extubation (15%), one for pain (4%) and one dropped the protocol (4%).
The mean and median duration of OS were 8.16±2.03 and 9 (2-10) weeks, respectively, after tube feeding indication for 19 patients who had not been using a tube. Eighteen (95%) of these patients remained on oral supplementation for three weeks or more, 15 (79%) remained for eight weeks or more and none for twelve weeks or more. There was a significant difference between TF and OS with regard to the percentages of patients that remained for eight weeks or more on each respective nutritional therapy (x2=10.3; p=0.0013).
There was a trend towards an association between age and gastrointestinal toxicity/vomiting, demonstrating that adolescents and young adults presented higher grades of this symptom (R2=0.07; r=0.59; p=0.08).
Clinical and nutritional outcome analyses
Analyses of supplement intake
The analysis for the percentage of supplement intake before TF indication (period 1) demonstrated mean and median of 51%±26 and 56% (0-100), respectively, of the recommended amount, without differences between TF and OS in period 1. This intake represented a mean of 24%±18 and median of 22% (0-80), of the TEE.
The mean percentage of supplement use of the TF group was different from that of the OS group during period 2 (Figure 2). There was also a statistical difference in supplement intake in the TF group, comparing the period with OS (period 1) and the period with TF (period 2) (Figure 3).
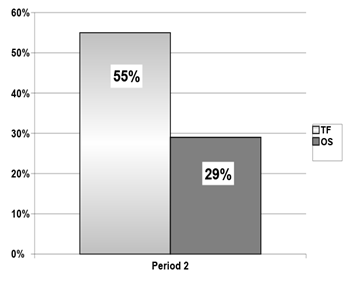
Figure 2 Median of supplement intake relative to total energy expenditure (TEE) during the tube feeding period (period 2): tube feeding regimen versus commercially-prepared oral supplementation.
TF: mean = 62%±35 and median = 55% (15–175); OS: mean = 23±12 and median = 29 (3–39) (p<0.00002; Mann-Whitney).
TEE, total energy expenditure; TF, tube feeding; OS, commercially-prepared oral supplementation
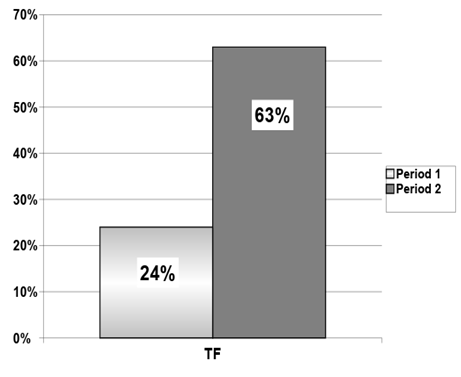
Figure 3 Comparison of the percentage supplement intake relative to total energy expenditure (TEE) between period 1 and period 2 in the tube feeding regimen.
Mean for period 1: 24±22; mean for period 2: 63±36 (p = 0.000004; paired Student t test) and median for period 1: 18 (0 – 80); median for period 2: 55 (15 – 175).
TEE, total energy expenditure; TF, tube feeding.
Tube feeding supplement analyses
There were statistically significant positive nutritional outcomes in relation to TSFT, MUAC and AMC, comparing week zero to the last week with TF (Figure 4).
The nutritional outcomes measured by TSFT, MUAC and AMC in the three distinct periods (period 1: three weeks with oral supplementation preceding TF; period 2: three weeks with TF; and period 3: three weeks after tube loss, receiving oral supplementation) demonstrated a significant positive outcome only in the TF period (TSFT: p = 0,012; MUAC: p = 0,002; AMC: p = 0,002).
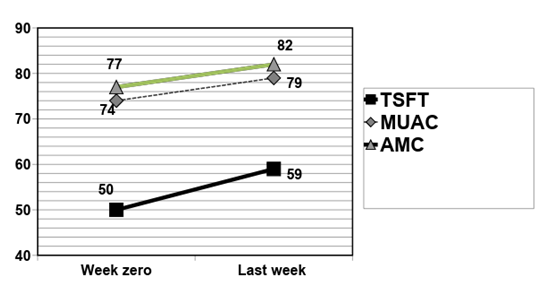
Figure 4 Nutritional evolution as a percentage of ideal triceps skin fold thickness (TSFT), mid-upper arm circumference (MUAC) and arm muscle circumference (AMC) between week zero and the last week with tube feeding regimen (n=23).
Statistical analysis (non-normal distribution: Wilcoxon test; values are represented by medians):
TSFT: week zero: 50 (31–100), last week: 59 (29–114); p=0.001; MUAC: week zero: 74 (58–87), last week: 79 (65–99); p=0.0002; AMC: week zero: 77 (64–90), last week: 82 (67–102); p=0.0004.
TSFT, triceps skinfold thickness;MUAC, mid-upper arm circumference; AMC, arm muscle circumference
The mean of leukocyte, platelet and hemoglobin concentrations comparing period 1 (OS) and period 2 (TF) did not demonstrate any difference in the TF group.
There was an association between the length of stay with the TF regimen (in weeks) and improvements of the nutritional status. The longer the period on TF, the higher the increase in percentage of nutritional adequacy (zero to the last week): TSFT (R2=0.72; r=3.1: 1.51–3.37; 95% CI; p=0.0002), MUAC (R2=0.80; r=1.35: 0.99–1.61; 95% CI; p=0.000001) and AMC (R2=0.73; r=0.98: 0.75–1.37; 95% CI; p=0.00003), when adjusted for diagnosis, age and educational level.
There was a significant inverse association between age and length of stay with TF regimen (R2=0.17; r=-0.36: p=0.049), suggesting that the greater the age was, the shorter the duration was. There were no associations between duration of the TF regimen and gender, educational level or diagnosis.
Analyses of the clinical and nutritional markers for the TF regimen during period 2 demonstrated a clinical trend of positive association between leukocytes and differences in percentage of the ideal AMC (R2=0.15; r=7.77; p=0.08), without other associations.
Tube feeding regimen versus commercially prepared oral supplementation
There were no statistical differences in median values of TSFT (2.3% and 2.45%) and MUAC (2.28% and 1.83%) between the TF and OS groups (respectively). A trend towards a difference was observed for AMC (2.72% and 1.17%; p=0.07).
Analyses of clinical markers demonstrated a non-significant higher mean for leukocytes (4596 versus 4486 cells/mm3) and platelets (245732 versus 225744 cells/mm3) in the TF group, but a statistically significant higher mean for hemoglobin (9.5 versus 10.5g/dl) in the OS group (p = 0.047).
Gastrointestinal toxicity (vomiting and diarrhea) was not associated with any nutritional index and there was no association between gastrointestinal intolerance and the route of supplement intake (TF versus OS) in period 2.
There was no difference in the number of episodes of hospitalization between the TF and OS groups, either in period 1 or in period 2.
The association between the year of patient admission into the protocol and the compliance with tube feeding was significant: those enrolled in 2002 were more likely to be compliant with the TF regimen than were those enrolled in 2003 (OR=5.0: 1.18-21; 95% CI; p=0.03). This analysis was adjusted for age, educational level, gender and diagnosis of cancer. The percentage of compliance in 2002 was 81%, versus 48% in 2003.
Several groups of experts have proposed protocols to identify patients at risk of malnutrition and to provide early nutritional support.26,31,32,40 In order to standardize this practice and to study this therapy, we developed an algorithm for enteral nutritional support decision-making.
Our study found that a high percentage of patients had an indication for TF, according to the protocol algorithm. A high percentage of them refused the TF, with an association between age and TF use. This could be related to cultural and psychological traits, taking into consideration evidence from the United States that parent refusal of treatment is a problem in pediatric oncology.41 The lack of knowledge of parents and patients regarding the prognostic importance of nutritional status during treatment may also negatively influence the decision to accept or not support nutritional support. In general, Brazilian healthcare teams in pediatric oncology are still resistant to many invasive support procedures. Despite this, there is no data on this practice in children with cancer in Brazil.
Another aspect of compliance was the length of time for which patients remained with the TF. Although 30% of them kept the tube for eight weeks or more, only 15% kept the TF until they had recovered from their malnutrition. Chemotherapy toxicity leading to vomiting among a high percentage of patients was probably the main cause of extubation in our study. In this study, older patients demonstrated shorter duration of TF and presented higher grades of gastrointestinal toxicity/vomiting. This could be related to the more aggressive anticancer protocols that were being used to treat patients with highly prevalent tumors during adolescence. In fact, 43.5% of the patients on a TF regimen were diagnosed with bone tumors, particularly osteosarcoma. Its treatment is based on high doses of cisplatin, which is a potent emetic agent.
Various studies have proposed a period of TF of around three to six months. Prolonged length of enteral feeding may ensure greater improvement of nutritional status.42–45 In the current study, there was an association between TF duration and nutritional indicators from week zero to the last week. However, some authors have recommended feeding by means of gastrostomy if the nutritional therapy has to be maintained for more than three months in pediatric cancer patients.26,32 Although eight weeks was proposed in our protocol, it was noted that one patient required TF for a longer period, but none used gastrostomy.
It was observed that the patients receiving OS remained on nutritional support for longer periods than did the patients with the TF regimen, and the difference between the patients in these two groups was significant in relation to attaining eight weeks or more. Nonetheless, the supplement intake was significantly higher with TF than with OS. On the other hand, higher rates of vomiting were associated with higher supplement intake using TF.
These data, and the high percentage of patients that had an indication for TF in our study support some other authors’ reports that oral supplementation may not be very effective in recovering nutritional status among children with cancer, since children do not accept oral dietary supplementation as an adequate means for reaching this objective.20,21 Thus, TF could be more effective for this purpose. Bakish et al.23 also reported evidence that children with cancer receiving TF showed better improvement of nutritional status than did those receiving oral diet counselling.23 The results from the present study have confirmed this evidence, taking into consideration the observation that there was positive nutritional evolution in the TF group, in intra-group analyses. Even the analysis at three weeks, which was a short duration of support, demonstrated an improvement in the nutritional status. This improvement was greater than what was obtained during other periods with OS alone. On the other hand, although there was no association between percentage supplementation achieved by tube feeding and the nutritional index, gastrointestinal toxicity with vomiting influenced tube feeding supplement intake.
Several studies indicate a positive evolution with the probe on nutritional status in pediatric patients with cancer It is well known that tube feeding is cheaper and safer than parenteral nutrition. Taken together, these data suggest that tube feeding could be the preferred route for feeding malnourished pediatric cancer patients.19,25 However, interpretations of these studies among children and adolescents with cancer are difficult because of study design limitations relating to numbers of patients, non-randomized trial designs, different diagnoses, concomitant parenteral nutrition and limitations on nutritional assessment methods.2,24,25,43–47 Furthermore, these studies did not describe the amount of tube feeding supplement intake.
Non-important differences in nutritional outcome were found between the TF regimen and OS in this study. It is difficult to assert anything regarding the analyses performed between groups that were not randomized to different treatments. Nonetheless, there were no statistical differences concerning gender, age, educational level, diagnosis, episodes of hospitalization or gastrointestinal toxicity.
The OS group may have been so worried about having the tube inserted that these patients decided to consume larger amounts of food and supplementation in order to avoid this. Another point is the finding that the patients who accepted the TF regimen were more severely malnourished and, therefore, it will have been easier to persuade them to use the TF.
In the present study, the main causes for indicating parentral nutrition and formula alterations were gastrointestinal problems from cancer therapy. However, differences in gastrointestinal toxicity between TF and OS were not observed, and this variable did not influence any nutritional index.48–50
Although the nutritional indices improved, the clinical markers did not demonstrate major differences between tube feeding and oral supplementation. Most probably, the nutritional support was not maintained for a long enough period to promote changes in this respect. Because of the enormous influence of other variables on these prognostic indexes, they were not sufficiently reliable to represent good markers in this study design. Important points that should be considered for future studies include. The investigation of the use of overnight pump infusion, specific antiemetic and prokinetic agents and increased use of nasoduodenal and nasojejunal feeding, as well as gastrostomy.
The primary limitation of the present study was its non-randomized design. Another type of limitations were the lack of oral diet intake analysis and the variability of the sample (diagnosis, age and degree of malnutrition).
However, in the current literature, few studies have an adequate methodological design. Several are retrospective, and all of them present great variability in diagnosis, age and the weight used as the nutritional index. Most of them had small numbers of patients and did not adequately describe the patients who refused tube feeding or what criteria were used for starting tube feeding.24,25,42,44,46,51 Some difficulties found in these studies relate to non-ethical aspects of randomization, considering that there is a general belief that tube feeding is more effective in improving nutritional status than is oral feeding.
Considering that enteral is more helpful than parenteral nutrition for patients on anticancer treatment.52–54 more research should be conducted along these lines in order to elucidate the prognostic benefits of enteral TF, leading parenteral route to special situations.55
Enteral feeding in pediatric oncology patients is an effective and safe method to affect weight positively. However, further research is needed for developing treatment guidelines, including establishing a timeline for initiation of feeding, and determining which patients are most likely to benefit from enteral feeding Hence, for a more precise interpretation of the results, a multicenter study is warranted in order to follow up a larger number of patients, with a periodic complete nutritional assessment and with enteral protocols included as part of the anticancer protocols.56–61
The TF protocol improved supplement intake and nutritional status, without enteral feed-related gastrointestinal complications. The duration of support was an important variable associated with nutritional outcome.
AG designed the study, coordinated and collected the data, performed the statistical analysis and drafted the manuscript. FAL and ASP critically evaluated the final version of the manuscript. All authors read and approved the final manuscript.
Authors would like to thank Renata Mentone and Nestlé Clinical Nutrition for support the supplement and the dietitians who participated in the nutrition team during the study, assisting in data collection: Priscila dos Santos Maia e Fernanda Rodrigues Alves.
The authors declare that they have no competing interests.
References of the protocol publicated article: Garófolo A, Maia PS, Petrilli AS, Ancona-Lopez F. Outcomes of the implementation of an enteral nutrition algorithm in children and adolescents with cancer. Braz J Nutr./Rev Nutr., Campinas, 23(5):715-730, set./out., 2010.
Methods
Study design
Oral and tube feeding regimen
Parents were encouraged to provide the prescribed volume and to call anytime if problems occurred. To maximally compensate for interruptions in feeding due to planned medical procedures, the infusion rate was temporarily increased, when this was tolerated, or the period of tube feeding was extended by a few hours on the day(s) before the procedure. The patient was advised to return to the hospital if the tube was lost due to inadvertent extubation at home, in order to have it reinserted.
Energy recommendation
In order to assess the adequacy of energy requirements and compare groups, total energy expenditure (TEE) was calculated using the following equation: baseline energy expenditure × [recovery factor, activity factor (moderate activity: 1.2), illness factor (1.3) and a factor for the thermal effect of food (1.1)]. The baseline energy expenditure (kcal/24h) according to age, weight and gender was determined using the WHO equation for subjects below 15 years of age and the Harris-Benedict formula for those over 15 years of age 33,34,35,36.
Results
Descriptive flow of Figure 1.
One hundred and seventeen patients out of 141 completed three weeks of follow-up; 47 had an indication for tube feeding (40.5%): 42 in week three and five in week eight. Three out of 47 had to be excluded from the study: one due to intestinal resection and the need for exclusive parenteral nutrition, one who refused to continue the follow-up and one who was not in an adequate clinical condition to be evaluated, due to cancer progression.
Therefore, 44 patients had an enteral TF indication and were analyzed: 27 (61%) consented to use the TF, but 17 (39%) did not. Out of the 27 who consented, four did not actually use the TF, because the tube became dislodged just after it had been inserted, and they were followed in OS group. Among the 17 patients that refused to use TF, two received appetite stimulants drugs and were excluded from the nutritional outcome analyses. Therefore, a total of 42 individuals were followed for the nutritional outcomes: 23 in the TF group and 19 in the OS group. Figure 1 shows this descriptive analysis in detail.
The main causes of non-acceptance of the TF regimen among the 17 subjects were patient refusal (53%), parent rejection (35%) and non-acceptance from the clinical team, with preference for the use of appetite stimulants (12%).
The analysis of the 23 patients who received TF demonstrated that 18 of them had the tube inserted within the same week that the TF indication was done, but nine were placed later. The primary causes for this delay were low platelet count (n=2), grade 3 mucositis (n=1), non-attendance at the nutritional consultation (n=1) and septic shock (n=1).

©2019 Garófolo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.