eISSN: 2576-4497


Online Report Volume 7 Issue 1
University of Brasilia, Brazil
Correspondence: Carmen Déa Ribeiro de Paula, University of Brasília Hospital and Brasília University Center, Quadra SHIN QI 12 Conjunto 7 10, Brazil, Tel +556120285427
Received: January 30, 2024 | Published: February 12, 2024
Citation: de Paula CDR. Carcinogenesis in epidermolysis bullosa: why use losartan for prophylaxis of squamous cell carcinoma? Hos Pal Med Int Jnl. 2024;7(1):9-10. DOI: 10.15406/hpmij.2024.07.00230
The risk of developing squamous cell carcinoma (SCC) in the general population is 1% in the 50-59 age group. When risk factors are not present, local recurrence occurs in 3%, lymph node metastasis in 4% and death due to SCC can occur in up to 1.5%. If, on the other hand, risk factors are present, local tumor recurrence is expected in up to 47.2%, local and distant metastasis in 47.3% and metastatic disease leading to death in up to 70%. Risk factors include tumor size and location, histological findings such as perineural and/or vascular invasion, and the degree of un-differentiation.1 Patient-related factors are also very relevant (Figure 1).
People with epidermolysis bullosa congenital (EB), especially the junctional and recessive dystrophic types, have an increased risk of developing cancers of epithelial origin, mainly SCC, but also basal cell carcinoma (BCC). The risk increases with the severity of the disease and the chronicity of the ulcer,2 reaching 90.1% of patients with recessive dystrophic epidermolysis bullosa (RDEB) by the age of 55. The table below summarizes the main epidemiological data of these tumors in patients with EB (Figure 2). As for the risk factors for metastasis, patients with EB have chronic skin inflammation (Figure 1). Thus, we can see from the figures that the more severe forms have a higher risk of SCC, as well as a higher risk of unfavorable evolution, such as metastatic disease and mortality.1
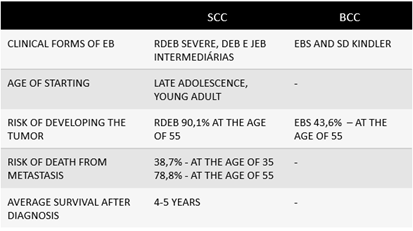
Figure 2 Epidemiological aspects of epithelial tumors.
SCC, squamous cell carcinoma; BCC, basal cell carcinoma; in patients with EB, epidermolysis bullosa; and its subtypes – RDEB, recessive dystrophic epidermolysis bullosa; DEB, dystrophic epidermolysis bullosa; JEB, junctional epidermolysis bullosa; EBS, epidermolysis bullosa simplex.
The most aggressive cases of SCC are found in the recessive dystrophic subtype (RDEB), characteristically the most mutilating form, with joint contractures, hardening of the skin and functional impairment of the limbs. The dystrophic forms are all attributed to mutations in collagen VII (COL7A1). This is the main component of the anchoring fibrils (AF), which can be morphologically altered, expressed or even absent in DEB. Nystrom,3 in 2013, using animal models, mice that did not express collagen VII (Col7a1 knockout - KO-mouse), demonstrated that collagen VII is essential for wound healing. The KO animals took much longer to epithelialize. It was shown that collagen was required for this process by 2 mechanisms. First: COL7A1 is necessary for the organization of laminin 332. The laminin 332/integrin α6β4 axis signals the migration of keratinocytes in the healing process. In the absence of COL7A1, laminin 332 loses its “organization”. This aberrant organization negatively modulates integrin α6β4 signaling in basal keratinocytes. Second: COL7A1 is essential for the migration and regulation of dermal fibroblasts that form granulation tissue. KO mice have high expression of TGFβ in fibroblastos.3
Collagen VII acts by regulating the composition of the extracellular matrix. One of the ways in which collagen VII regulates this is by inhibiting the expression of TGF-β. Increased TGF-β activity contributes to the fibrosis phenotype.4 chronic inflammation is a stimulus for TGF-β activation. In a patient with normal COL VII production, this activation would be controlled. In patients with EBDR, the constant process of damage and repair leads to the production of inflammatory cytokines and increased activation of TGF-β. In diseases with a fibrosis phenotype, TGF-β (Transforming growth factor beta) plays a central mediating role. Thrombospondin 1 (Tsp1), an extracellular matrix protein that responds to tissue injury, is the most widely studied TGF-β activating factor. In order to activate latent TGF-β, Tsp-1 breaks the link between the N-terminus of LAP (latency-associated peptide) and the C-terminus of the mature domain. In addition to Tsp-1, integrin’s and proteases also activate TGF-β. Blocking the Tsp-1/TGF-β axis may be a pathway in fibrosing diseases.5
Ng et al,6 showed that the fibroblasts present in the skin under SCC in patients with RCDB allowed for greater adhesion and invasion of keratinocytes. The same authors showed an increase in collagen XII, thrombospondin-1 and Wnt-5A protein in the fibroblasts of people with RCDB. Re-expression of the wild-type COL7A1 gene in EBDR fibroblasts led to a reduction in collagen XII, thrombospondin-1 and Wnt-5A, as well as a reduction in tumor cell invasion and tumor growth.6
Losartan, an angiotensin II receptor-blocking drug (ARB), has been widely used as an antihypertensive agent since 1995, when it was approved by the FDA. It is also indicated in myocardial hypertrophy and chronic kidney disease. Losartan has been shown to improve myocardial fibrosis by reducing the expression of TGF-β. In an in vitro study, losartan reduced levels of TGF-β in cultured cells from a patient with RDEB and in vivo in the skin and circulation of mice with RDEB (C7-hypomorphic mice: these animals express 10% of normal collagen VII). The animals given losartan showed less fibrosis and a slower progression to digit fusion. There was also a reduction in tenascin-C and fibronectin, two markers of fibrosis, in the paws of the animals treated with losartan.3
Losartan can reduce TGF-β signaling by mechanisms such as: blocking the angiotensin II type 1 receptor, which reduces the expression of latent TGF-β activators such as Tsp-1 or by reducing the expression of TGF-β ligands and their receptors.3 A study evaluating the efficacy and safety of losartan for children with RDEB and esophageal stenosis showed that, in the group receiving the medication, the recurrence of stenosis was much lower (one child in 9, while in the control group there were 4 children in 10), as well as the malnutrition rates and severity of EB were higher in the control group.7
Patients with RDEB have reduced or absent COL7A1. Phenotypically, they have significant fibrosis. As studies have shown, one of the factors contributing to fibrosis is the high expression of TGF-β. Cutaneous squamous cell carcinoma that settles on the altered dermis of dystrophic epidermolysis bullosa becomes more aggressive and invasive. Losartan, a drug that has already been used as a modulator of TGF-β levels, reducing esophageal stenosis and skin fibrosis, may also be able to delay deformities and even the aggressiveness of SCC, since it reduces TGF-β signaling and thus the alteration of fibroblasts found in RDEB. To date, there are no treatments for EB. The intervention of health professionals is limited to care: prevention of blisters, healing, reduction of pain and itching. Early recognition of complications, including SCC, has been widely emphasized. Drugs with the potential to reduce these complications deserve to be further studied.
None.
The author declare that there is no conflicts of interest.

©2024 de. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.