eISSN: 2576-4462


Research Article Volume 6 Issue 1
1Laboratorio de Microbiología Ambiental, Mich, Mexico
2Centro de Investigaciones Químicas, IICBA, Universidad Autónoma del Estado de Morelos, Av Universidad 1001, Col, Chamilpa, Cuernavaca, Morelos 62209, Mexico
3Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, Massachusetts 02138, United States
Correspondence: Juan Manuel Sánchez-Yáñez, Laboratorio de Microbiología Ambiental, Instituto de Investigaciones Químico Biológicas, Ed-B3, Universidad Michoacana de San Nicolás de Hidalgo, Francisco J Mujica S/N, Col Felicitas del Rio CP, 58000
Received: December 17, 2021 | Published: February 17, 2022
Citation: Velázquez-Medina A, Cruz JLID, Lopez N, et al. Triticum aestivum response to Xanthobacter autotrophicus and Bacillus thuringiensis optimized by crude extract carbon nano particles and 50% of NH4 NO3. Horticult Int J. 2022;6(1):31-35. DOI: 10.15406/hij.2022.06.00239
Triticum aestivum requires nitrogen fertilizer (NF) as NH4NO3 for healthy growth, however its excessive application causes loss of soil fertility. An alternative to decrease the amount of NH4NO3 and to optimize is to inoculate T. aestivum seeds with Xanthobacter autotrophicus or/and Bacillus thuringiensis well known asendophytic plant growth promoting bacteria (EPGPB). Both genera and species of EPGPB are able transform metabolic compounds from seeds and roots into phyto hormones, which can be optimized by crude extract carbon nano particles (CECNPs) to enhance the growth of radical system and to improve uptake at 50% dose of NH4NO3 Response variables were: germination percentage, phenology, and biomass to seedling stage. All experimental data were validated by ANOVA/Tukey HSD P<0.05. Seeds of T. aestivum were inoculated with X. autotrophicus and/or B. thuringiensis, plus CECNPs at 50% NH4NO3. Results showed a positive response of T. aestivum seeds to X. autotrophicus and/or B. thuringiensis, with 10 ppm CECNPs, and 50% NH4NO3 enhanced germination percent of 93% in comparison with 73% of T. aestivum when its seeds were not inoculated with these EPGPB fed only with 100% dose of NH4NO3 (relative control); the same positive response of T. aestivum to X. autotrophicus and B. thuringiensis improved by CECNPs at seeding stage compared to T. aestivum used as a relative control.
Keywords: soil, nitrogen fertilizer, wheat, EPGPB, CECNPs, phytohormons
The healthy growth of Triticum aestivum (wheat) requires nitrogen fertilizer (NF) such as NH4NO3,1 but applied in excess leads to cause of soil productivity due to accelerate mineralization of the organic matter.2 An alternative ecological solution to this problem related to NF, is to inoculate seeds of T. aestivum with X. autotrophicus and B. thuringiensis both genera and species are endophytic plant growth promoting bacteria (EPGPB), its beneficial effect can be optimized with a crude extract of nano particles (CECNPs) at 50% dose of NH4NO3.Due to X. autotrophicus and B. thuringiensis are able to transform seed exudates and metabolic compounds from photosynthesis of roots into phytohormones, to improve germination and to induce accelerated and dense root formation that optimizes uptake reduced dose of NH4NO3, without detrimental effects on the healthy growth of T. aestivum.3-7 Currently, there is scarce information on the optimized of the beneficial effect of X. autotrophicus and B thuringiensis to maximize the capacity of radical absorption and optimization at 50% of NH4NO3 this beneficial activity of EPGPB can been hancing by applying a CECNPs, to improve the germination and plant growth, as reported by Srivastava & Rao,8 whom treated Zea mays seeds with 5 ppm of multi-walled carbon nano tubes (MWCNTs), where up to 80% germination was recorded compared to 60% of Z mays without the MWCNTs irrigated only with water. While Joshi et al.,9 analyzed the effect of T. aestivum with 8 ppm nano particles of carbon, increased aerial fresh weight of 1.3 g and 0.09 g of root fresh weight in comparison with aerial fresh weight of 0.8 g and 0.5 g of root fresh weight obtained for treatment with only 100% NH4NO3 dose. In that sense the aim of this research work was to analyze Triticum aestivum with X. autotrophicus and/or B. thuringiensis, enhance by CECNPs, at 50% of NH4NO3.
This research at the greenhouse of the Environmental Microbiology laboratory - Instituto de Investigaciones Químico-Biológicas at Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mich., México. The studies were performed under greenhouse environmental conditions with average values: T = 23.2°C, luminosity = 450 µmol•m-2•s-1, relative humidity = 67%. The agricultural soil was collected from 19°37’10” north latitude and 101°16’41.00” west longitude, with an altitude of 2013 meters above sea level, at a temperate climate zone called “Uruapilla” municipality of Morelia, Michoacán, Mexico, on the Morelia-Pátzcuaro highway. The soil was sieved with a mesh No. 20 and was exposed to sun light for 40 h to prevent pests and diseases,10 the physicochemical properties are shown in Table 2.
Leaves of A. pluriforma were collected from UMSNH campus in Morelia, Mich, México. The leaves were disinfected by immersion in a 0.5% NaClO for 1 min and were rinsed with sterile deionized H2O. The leaves were cut into 5 cm pieces and dried in an oven at 80°C for 12 h, 30 g were suspended in 300 mL deionized H2O, heated at 70°C for 30 min and filtered through a Whatman No.1 paper. The filtrate was centrifuged at 4000 rpm for 10 min and then placed in a fridge at 4°C. Subsequently for the characterization of the CECNPs a JEOL JSM-6400 scanning electron microscope (SEM) from the Institute of Research in Metallurgy and Materials of the UMSNH, Morelia, Mich, Mexico was used to characterize the size and morphology of the nano particles synthesized from A. pluriforma according to the following procedure: dried at 100°C/12 h, then a gold film was applied to the CECNPs to allow the sample to be conductive so that the size of the nanoparticles could be analyzed. To determine the qualitative and quantitative composition of CECNPs was performed by energy dispersive spectroscopy (EDS), for this the CECNPs was dried, then analyzed using a JEOL-JSM-7600F EDS field emission microscope.
T. aestivum seeds had a positive response to X. autotrophicus and B. thuringiensis, enhancing with CECNPs at 50% of NH4NO3. T. aestivum seeds were disinfected with 0.2% NaClO for 5 min, rinsed 6 times with sterile tap water and disinfected then with ethanol 70% (v/v) for 5 min. The seeds were rinsed 6 times with sterile tap water and inoculated with X. autotrophicus and/or B. thuringiensis.10 A suspension of saline solution 0.85% of NaCl (wt/v) of 1.0 mL de X. autotrophicus and/or B. thuringiensis in a relation 1:1 (v/v) was used for every 10 seeds of T. aestivum, followed by treatment with 1.0 mL of CECNPs at 10 or 20 ppm concentration, 0.85% NaCl and 0.5% (wt/v) of commercial detergent “La Corona”MR. The treated seeds were stirred at 200 rpm for 30min at 28°C to generate an homogeneous dispersion of CECNPs, then sown in a container with 100 g of soil (Table 2), fed with a mineral solution (MS) with recommended dose of NF as NH4NO3 for T.aestivum as nitrogen source of following chemical composition (g/L): NH₄NO₃ 10; K2HPO4, 2.5; KH2PO4, 2.0; MgSO₄ 0.5; NaCl, 0.1; CaCl2, 0.1; FeSO4 1.0; and a microelements solution (g/L): H₃BO₃ 2.86; ZnSO₄•7H₂O 0.22; MgCl₂•7H₂O 1.8, at pH = 6.8. The MS was applying to feed T. aestivum using a volume of 5 mL every 3 days per month to assure the field capacity at 80%. The test was carried out according to Table 1, which shows the experimental design of random blocks with 6 treatments, 2 controls and 10 repetitions, with the following conditions: Absolute control (AC), T. aestivum irrigated with water only; Relative Control (RC): T. aestivum fed with100% NH4NO3 without X. autotrophicus and/or B. thuringiensis and all treatments: T. aestivum with X. autotrophicus and/or B. thuringiensis, plus 10 or 20 ppm CECNPs, at 50% NH4NO3.The variables-responses of this test were germination percentage (%) between four and ten days after sowing, phenology: plant height (PH) and root length (RL); and the biomass: aerial fresh weight/root fresh weight (AFW/RFW) and aerial dry weight/root dry weight (ADW/RDW) at seedling were analyzed at 30 days after sowing. The results were validated by ANOVA/Tukey HSD P<0.05% using the Statgraphics Centurion software.11
|
Triticum aestivum* |
NH4NO3 (%) |
Crude extract carbon nanoparticles |
Xanthobacter autotrophicus |
Bacillus thuringiensis |
|
Pure water, absolute control (AC) |
0% |
- |
- |
- |
|
NH4NO3, relative control (RC) |
100% |
- |
- |
- |
|
T= 1 |
50% |
10 ppm |
- |
+ |
|
T= 2 |
50% |
10 ppm |
+ |
- |
|
T= 3 |
50% |
10 ppm |
+ |
+ |
|
T= 4 |
50% |
10 ppm |
- |
- |
|
T= 5 |
50% |
20 ppm |
- |
+ |
|
T= 6 |
50% |
20 ppm |
+ |
- |
|
T= 7 |
50% |
20 ppm |
+ |
+ |
|
T= 8 |
50% |
20 ppm |
- |
- |
Table 1 Experimental design of the response of Triticum aestivum to Xanthobacter autotrophicus and Bacillus thuringiensis with a crude extract carbon nano particles and 50% NH4NO3 dose
*n = number of repetitions = 10; T = treatment; inoculated with EPGPB (+); uninoculated (-)
|
Parameter |
Value /Interpretation |
|
pH (1:20) |
5.75 moderate acid |
|
Organic matter |
10.44% high |
|
Cationic exchange capability |
11.16 Cmol/Kg not saline |
|
Electrical conductivity 1:2 (H2O) |
0.91 mS/cm not saline |
|
Texture |
Percentage (%) clay 31.0,slit 42.0,sand 26.92/ clayey slit |
|
Apparent density (g/cm3) |
0.72 g/cm3 |
|
Total nitrogen |
0.32 % / medium |
|
Nitric nitrogen (ppm) |
244.16 ppm / medium |
|
Phosphorous (PO4- ppm) |
219.34 ppm / medium |
|
Humidity |
13.25% / low |
Table 2 Physicochemical properties of soil inoculated with Xanthobacter autrotrophicus and/or Bacillus thuringiensis, treated with crude extract carbon nano particles and 50% NH4NO3 dose
Table 2 shows the physicochemical properties of soils, with analysis according to the Mexican regulation NOM-021-RECNAT-2000. The soil is slightly acidic (pH = 5.75), with high content of organic matter of 10.44%, with texture: clay 31.08%, slit 42.00%, sand 26.92%, and classified as clay-loam soil. It contains a medium concentration of total N of 0.32%, 244.16 ppm NO3-, and 219.34 ppm PO4-.
Figure 1 shows the SEM micrographs providing the morphology and size of the CECNPs nano particles synthesized from A. pluriforma, which presented spherical shapes with variable size, where some tended to be dispersed while others formed aggregates (Figure 1a). Additionally, EDS analysis provided a qualitative and quantitative status of the elements that may be involved in the formation of these nano particles. The elemental profile of the CECNPs recorded an atomic percentage of carbon of 50.98%, being the main element. There was also presence of oxygen up to 30.41% atomic and some traces of magnesium, phosphorus, chlorine and potassium, confirming the formation of carbon nano particles (Figure 1b).12
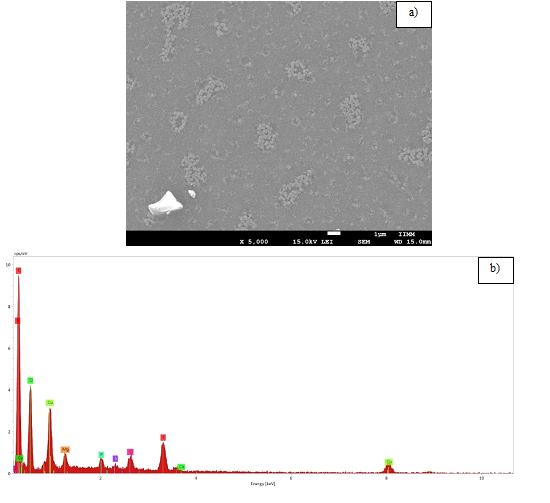
Figure 1 Morphology and qualitative/quantitative analysis of CECNPs from Albizia pluriforma:
a. SEM micrographs and
b. EDS composition.
Table 3 shows 73% of germination of T. aestivum seeds for the relative control, 93% of germination of T aestivum seeds with B. thuringiensis and X. autotrophicus with 10 ppm CECNPs and 50% NH4NO3, while increasing the CECNPs concentration to 20 ppm causes a decrease in germination to 86%. This suggests that water uptake by the seeds induced the activity of α-amylase that hydrolyzes amylose with the release of organic acids, amino acids and sugars,12 that both B. thuringiensis and X. autotrophicus transformed in gibberellin-type phytohormons which were enhancing by the CECNPs accelerated the interruption of dormancy in the seeds.9,14-16
|
*Triticum aestivum |
Germination (%) |
|
Pure water, absolute control (AC) |
66c** |
|
NH4NO3 100% orrelative control (RC) |
73b |
|
B. thuringiensis, 10 ppm crude extract carbon nanoparticles (CECNPs) and 50% NH4NO3 |
76b |
|
X. autotrophicus, 10 ppm CECNPs and 50% NH4NO3 |
76b |
|
B. thuringiensis /X. autotrophicus,10 ppm CECNPs and 50% NH4NO3 |
93ª |
|
10 ppm CECNPs and 50% NH4NO3 solution |
80b |
|
B. thuringiensis, 20 ppm CECNPs and 50% NH4NO3 |
80b |
|
X. autotrophicus, 20 ppm CECNPs and 50% NH4NO3 |
86a |
|
B. thuringiensis /X. autotrophicus, 20 ppm CECNPs and 50% NH4NO3 |
40d |
|
20 ppm CECNPs and 50% NH4NO3 |
46d |
Table 3 Seed germination percentage of Triticum aestivum with Xanthobacter autotrophicus, Bacillus thuringiensis crude extract carbon nanoparticles and 50% NH4NO3 dose
*n=10**Different letters indicate statistical difference by ANOVA/Tukey HSD P<0.05%
Table 4 shows the phenology at seedling stage of T. aestivum with X. autotrophicus, B. thuringiensis, 20 ppm CECNPs and 50% of NH4NO3, where it registered 20,5 cm plant height (PH) and 16,16 cm root length (RL), numerical values with statistical difference compared to the 18,85 cm PH and 10,83 cm RL of T. aestivum without inoculating X. autotrophicus, B. thuringiensis, 20 ppm CECNPs with 100% of NH4NO3 as a nitrogen fertilizer (NF) or relative control (RC). Related to biomass of T. aestivum inoculated with X. autotrophicus, B. thuringiensis, plus 20 ppm CECNPs and 50% of NH4NO3 registered 0.34 g aerial fresh weight (AFW), 0.17 g root fresh weight (RFW), 0.07 g aerial dry weight (ADW) and 0.036 g root dry weight (RDW). All these numerical values showed statistical difference compared to T. aestivum without X. autotrophicus and/or B. thuringiensis, not treated with CECNPs at 100% of NH4NO3 or relative control (RC) with following values: 0.21 g AFW, 0.11 g RFW, 0.02 g DAW and 0.020 g RDW of It is possible that CECNPs improved the activity of X. autotrophicus and/or B. thuringiensis, converting organic compounds of the root and photosynthesis metabolism into phytohormons that induce accelerate growth of hole roots system,2,17 enhancing by CECBPs that maximizing the radical absorption of 50% of NH4NO3.18-21 In Table 4 indicate that the positive responds of T. aestivum to B. thuringiensis and/or X. autotrophicus plus CECNPs enhancing dry aerial weight and dry root weighare higher than values observed for T. aestivum or relative control, which suggest that CECNPs improving the uptake of NH4NO3 as a nitrogen fertilizer without the risk of losing soil productivity and might prevent pollution of the environment caused by the irrational use of nitrogen fertilizer.
|
* Triticum aestivum |
Plant height (cm) |
Root length (cm) |
Fresh weight (g) |
Dry weight (g) |
||
|
Aerial |
Root |
Aerial |
Root |
|||
|
Pure water, absolute control (AC) |
15.33d** |
9.83e |
0.13d |
0.05d |
0.01d |
0.015d |
|
NH4NO3 100% or relative control (RC) |
18.5b |
10.83e |
0.21c |
0.11c |
0.02d |
0.020c |
|
B. thuringiensis, 10 ppm crude extract carbon nanoparticles (CECNPs) and 50% of NH4NO3 |
17.66c |
17ª |
0.26c |
0.20ª |
0.05b |
0.026b |
|
X. autotrophicus, 10 ppmCECNPs and 50% of NH4NO3 |
19.83b |
11d |
0.29c |
0.17b |
0.05b |
0.026b |
|
B. thuringiensis /X. autotrophicus,10 ppm CECNPs and 50% of NH4NO3 |
21.0a |
12.66d |
0.31b |
0.18a |
0.05c |
0.036ª |
|
10 ppm CECNPs and 50% of NH4NO3 |
18.33b |
13.83cd |
0.25c |
0.21ª |
0.05b |
0.033ª |
|
B. thuringiensis, 20 ppmCECNPs and 50% of NH4NO3 |
20a |
15.33b |
0.24b |
0.12b |
0.04c |
0.031a |
|
X. autotrophicus, 20 ppmCECNPs and 50% of NH4NO3 |
19.33b |
12.83d |
0.22c |
0.12b |
0.04c |
0.027b |
|
B. thuringiensis /X. autotrophicus, 20 ppm CECNPs and 50% NH4NO3 |
20.5a |
16.16a |
0.34ª |
0.17b |
0.07ª |
0.036ª |
|
20 ppm CECNPs and 50% of NH4NO3 |
18.33b |
15.66b |
0.27b |
0.18a |
0.06ª |
0.030a |
Table 4 Phenology and biomass of Triticum aestivum with Xanthobacter autotrophicus, Bacillus thuringiensis plus crude extract carbon nano particles and 50% of NH4NO3 as a nitrogen fertilizer
*n=10; ** Different letters indicate statistical difference by ANOVA/Tukey HSD P<0.05%
T. aestivum had a positive response to X. autotrophicus and B. thuringiensis enhanced by ECNPCs (T1-T8) on seedling stage of growth are visually evident (Figure 2). A largest stem diameter is observed in T. aestivum with X. autotrophicus and/or B. thuringiensis plus ECNPCs this fact suggests that X. autotrophicus and B. thuringiensis they convert compounds of the photosynthesis metabolism into phytohormons such as auxin to induce an accelerated formation of dense roots in this way the root system enhancing the exploration capacity increased the exploration capacity of the soil improved by CECNPs for the optimization of 50% of NH4NO3 in comparison to T. aestivum without any EPGPB, fed with 100% of NH4NO3regard as a relative control showing that normally T.aestivum not inoculated with EPGPB was unable to effectively absorb of NH4NO3 for better growth causing soil lost fertility and environmental pollution.9,13,17

Figure 2 Response of Triticum aestivum seedling to Xanthobacter autotrophicus and Bacillus thuringiensis enhanced with a crude extract carbon nano particle and 50% NH4NO3.
*n=10
AC = T. aestivum pure water
RC = T. aestivum without X. autotrophicus and 100% NH4NO3
T1 = B. thuringiensis, 10 ppm crude extract carbon nano particles (CECNPs), 50% of NH4NO3
T2 = X. autotrophicus, 10 ppm CECNPs, 50% of NH4NO3
T3 = B. thuringiensis/ X. autotrophicus, 10 ppm CECNPs, 50% of NH4NO3
T4 = 10 ppm CECNPs, 50% of NH4NO3
T5 = B. thuringiensis, 20 ppm crude extract CECNPs, 50% of NH4NO3
T6 = X. autotrophicus, 20 ppm CECNPs, 50% of NH4NO3
T7 = B. thuringiensis/ X. autotrophicus, 20 ppm CECNPs, 50% of NH4NO3
T8 = 20 ppm CECNPs, 50% of NH4NO3.
The positive response of T. aestivum to X. autotrophicus or mixed with B. thuringiensis enhances the uptake of 50% NH4NO3 without the risk of affecting the healthy plant growth. Future studies will be performed with purified crude extract carbon nano particles to figure out which carbon nanostructure is responsible for enhancing the health growth of T. aestivum. It is expected that the ongoing studies might lead us to combinations in which lower doses of nitrogen fertilizer could be applied without compromising healthy growth of T. aestivum and thus avoiding soil productivity and environmental pollution.
We acknowledge support from a Mexico Innovation Fund grant provided by the David Rockefeller Center for Latin American Studies at Harvard University (2020-2022). To the project 2.7 (2022) from CIC-UMSNH, BIONUTRA S. A. de C.V., Maravatío, Mich., México.
The author declares there is no conflict of interest.

©2022 Velázquez-Medina, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.