eISSN: 2576-4462


Research Article Volume 3 Issue 2
Department of Horticulture & Landscape Architecture, USA
Correspondence: Department of Horticulture & Landscape Architecture, Fort Collins, Colorado, 80523-1173, USA
Received: July 03, 2019 | Published: April 30, 2019
Citation: Getlawi AO, Shahba MA, Hughes HG. Screening glaucium species for drought resistance with emphasis on the contributing physiological characters and overall performance. Horticult Int J. 2019;3(2):100-113. DOI: 10.15406/hij.2019.03.00117
With increasing population demands on the world’s water supply, there is a greater need for drastic water conservation methods especially in arid and semiarid regions. Plant species, and cultivars within a species, vary in their salinity/drought tolerance. These variations are the result of variations in genes relating to drought tolerance mechanisms and their interaction with the environment. In order to reduce water usage, it is important to understand the mechanisms of plant adaptation to drought stress. Horned Poppies (Glaucium spp) are members of the Poppy family, Papaveraceae, that are native to the Mediterranean and Middle East regions. The objectives of this study were to
Lysimeter columns were used in this study which was replicated twice. All columns were placed in the Colorado State University plant science green house in Fort Collins, Co. Glaucium spp. were initiated from seeds sown in potting mix, (Pro-Mix, Mycorrhizae and Biofunglcide). Fifty seedlings, at the 3-leaf stage, of each species were transplanted, each per PVS tubes (15 cm diameter and 50 cm long) containing commercial potting mix. The potting mix was mixed with sand 2:1 to increase pore space. Water regimes applied included control (100% of the total ET), as well as 75%, 50% and 25% of the total ET. With lower water regimes, leaf color declined over time to unacceptable ratings (below 6) in both G. grandiflorun and G. corniculatum. In G. flavum, and G. acutidentatum, leaf color was not adversely affected under all water regimes. The effect of water stress on leaf color among all species was highly significant. The decline in leaf color was high for all species at 50% and 25% of ET. Leaf area decreased linearly in all species with increasing drought with a sharp drop at 25% of the total ET. G.flavum achieved the highest leaf area at all water regimes followed by G. acutidentatum, G. grandiflorum and G. corniculatum. G. flavum acheived an average leaf area of 24.3 cm2, while G. acutidentatum leaf area was 22.2 cm2 at 100% ET. G. flavum achieved an average height of 45.8 cm while G. acutidentatum was 40.5 cm and G. grandiflorum was 30.0 cm at 200% ET. G. corniculatum had the lowest height of 27.8 cm in the control treatment. Increased water stress resulted in fewer flower buds, reduced flower number, and smaller flowers in all tested species. Also, increasing drought decreased the attractiveness of all Glaucium spp. although at different degrees. G. flavum showed greater transpiration efficiency (TE) since it was able to maintain its ET at lower rates while maintaining higher attractiveness when compared with G. acutidentatum, G. grandiflorum and G. corniculatum in order of attractiveness, respectively. In G. flavum, as water regimes decreased from control to 75, 50 and 25 % of the total ET, average TNC decreased by 15.1, 30.3 and 48.0% and the average TNC decrease in G. acutidentatum shoots was 21.6, 40.1, and 53.7%. RSC response to drought treatments followed a different trend than TNC. As water stress increased from control to 75, 50 and 25%, average RSC increased by 40.7, 101.8 and 166.5 % in G. flavum and by 17.4, 40.0 and 103.4% in G. acutidentatum. The increase was 122.2, 39.6, and 90.6% in G. grandiflorum and 4.4, 26.5, and 62.5% in G. corniculatum, respectively. As water regimes decreased from control to 75, 50 and 25%, average proline content in shoots increased by 186, 325, and 472% in G. flavum; 163, 303 and 517% in G. acutidentatum; 160, 280 and 418% in G. grandiflorum, and 80, 190, and 340% in G. corniculatum, respectively. On the basis of the number of times in the best statistical category for leaf characteristics, plant height, flowering characteristics, overall plant quality (attractiveness), water use efficiency, TNC, RSC, and Proline, G. flavum was found to have higher drought tolerance as compared to G. acutidentatum, G. grandiflorum and G. corniculatum. In summary, as drought increased, Glaucium spp. exhibited reduction in leaf characteristics, plant height, flowering characteristics, overall plant quality (attractiveness), TNC, and ET rate, and increased shoot total reducing sugars and proline content. G. flavum showed higher drought tolerance at all water regimes when compared to the other tested species. Since proline accumulation increased with drought stress it is likely that it aided drought tolerance through osmoregulation or by acting as a carbon and nitrogen sink for stress recovery.
Keywords: horned poppies, drought tolerance, proline, glacium flavum, g. corniculatum, g. grandiflorum and g. acutidentatum
EC, Electrical Conductivity; TNC, Total Nonstructural Carbohydrate Content; RSC, Shoot Reducing Sugar Content; ET, Evapotranspiration Rate; TE, Transpiration Efficiency
The demand for water has increased more than 300% during the past five decades. With increasing population demands on the world’s water supply, there is a greater need for drastic water conservation methods especially in arid and semiarid regions. Because of this immense water usage and diminishing water resources, many arid states have implemented water conservation programs.1 The demand for water has led to an inadequate water supply for landscapes and as a result negative impacts on the aesthetics and functionality. Therefore, the development of efficient irrigation management programs as well as the selection and improvement of drought tolerant landscape plants has become extremely important to maintain quality landscapes. Plant species, and cultivars within a species, vary in their salinity/drought tolerance. These variations are the result of genes relating to drought tolerance mechanisms and their interaction with the environment.2 Usually evaluations for drought and salt tolerance of plants depend on shoot (above ground) growth, as reported in crop yield response curves proposed by Maas and Hoffman.3,4
Horned Poppies (Glaucium spp) are members of the Poppy family, Papaveraceae. Glaucium are species that have originated in the Mediterranean and Middle East regions. Some species have a wider distribution than others. Horned poppies require full sun and well-drained soils for optimum growth. They should be spaced between 30 and 60 cm apart and are best grown by seeding in the fall where they are to bloom and thinning to the desired spacing as they germinate in the spring. For earlier bloom, sow seed indoors 8 to 10 weeks prior to planting and then transplant them into the garden after danger of frost has passed. Germination takes 8 to 15 days at 15 to 18oC. Seedlings should be transplanted to individual pots when three leaves have formed but before the taproot has developed. Transplanting should be done without disturbing the root system. Stems of horned poppy branch and grow to from a rosette of leaves. The crinkly, gray-green leaves also appear on the stems and below each flower. The golden-yellow flowers may be up to 5 cm in diameter. There are also orange or red flowers. The roots of the horned poppy are considered poisonous.
All horned poppies have blue-green foliage that is deeply pinnatified to pinnatisect and typically grow 30-50 cm long. The leaves have varying degrees of texture from glaucous to villous. All leaves are lyrate to sublyrate shaped and have a rosette growth habit. They have solitary blooms on flower stalks that grow above the foliage. All species have four petals in their corolla and their pistil is surrounded by stamens. They all develop long horned-shaped seed siliquiforms with the stigma remaining to cap off the top of the fruit. Species of interest in this study were G. flavum, G. grandiflorum, G. acutidentatum and G. corniculatum.
G. flavum Crantz is the most widely spread species in the genus. It is found from the coasts of Britain and the Atlantic Islands to the coasts of the Mediterranean Basin and the Black Sea.5 It grows predominantly on sandy beaches and as a result it is commonly known as the Sea Horned Poppy. This likely indicates that G. flavum is salt tolerant as it grows along the sea. According to Davis,5 G. flavum is distinguished from other species by several characteristics. The sepals have crisp, pilose hairs on the surface and the petals can be solid yellow, red or reddish mauve. G. flavum is most often recognized for the yellow petals and is commonly referred to as the Yellow Horned Poppy. The ovary is densely papillose to tuberculate, basically a bumpy surface. The siliquae will retain the papillose to tuberculate texture. In Turkey, G. flavum normally flowers from May through the summer and even though it is most often found at sea level, it does grow into river valleys as well.6
G. grandiflorum Boiss & É. Huet is native to Turkey in the southern part of the Caucasus Mountains but it is also found in Syria, Iran and the Sinai.5 Turkey is situated between the Mediterranean Sea and the Black Sea, where the precipitation ranges from 580 to 1300 mm/year. However, in the mountain ranges of the country there are great differences in climate changes with harsh winters and drier conditions with low precipitation of 400 mm/year. G. grandiflorum has features that distinguish it from other Glaucium species. It has only one main flower stem while other species have multiple flower stalks growing from the base of the rosette.6 The sepals have short, stiff hairs making the surface hirsute. The petals are dark orange to crimson red with a black spot at the base of the petal. The pedicle of the flower exceeds the subtending leaf, which differs from the other Glaucium species. There are two varieties of G. grandiflorum: var. grandiflorum and var. torquatum. G. grandiflorum var. torquatum has red petals with a black blotch and can be found in calcareous hillsides. G. grandiflorum var. grandiflorum is found in fields, banks and rocky slopes.
G. acutidentatum Hausskn & Bornm is endemic to Turkey where it is found on dry hillslopes and rocky places.5 G. acutidentatum is the most glabrous species with smooth sepals and ovaries. Although the ovary is smooth, the resulting siliquae is subtorulose. The petals are solid orange-buff color. G. acutidentatum is found at elevations of 950-1400 m on dry hills.6 G. corniculatum (L.) J.H. Rudolph is native to the Mediterranean basin, Atlantic islands, Caucasus Mountains, Bulgaria, Romania, northern Iraq and northwestern Iran.5,6 G. corniculatum also has some unique characteristics. Its leaves have a soft, villous texture and its sepals are scabrous to hirsute. The petals are yellow, orange or red6 with a black basal spot.5
To reduce water usage, it is important to understand the mechanisms of plant adaptation to drought stress. Drought resistance includes a range of mechanisms employed by plants to withstand periods of drought.7 Strategic mechanisms include drought escape, drought avoidance, and drought tolerance.8 The significance of each of these strategies is related to drought duration and severity in addition to the plant species. These mechanisms are associated with anatomical, morphological, physiological, and biochemical changes. The reduction in the evapotranspiration (ET) rate and the ability of a species to maintain transpiration as the soil dries are example of drought tolerance mechanisms as the reduction in ET indicates a better water use efficiency. Changes in leaves that facilitate drought tolerance include reduced leaf growth and area, increased pubescence, rolling or folding, and fewer stomates.2 The balance between carbohydrate production and consumption will impact the ability of plant species to cope with stresses.9–13 Amino acids, especially proline, accumulate in larger amounts to cope with increasing stress in plants.11 Proline accumulation is one of the first responses of plants exposed to water-deficit stress and serves to reduce injury to cells.14 Rapid accumulation of proline in tissues of many plant species in response to drought, salt or temperature stresses has been attributed to enzyme stabilization and/or osmoregulation.15,16 However, because of contrasting reports related to proline accumulation effect on stress tolerance,17,18 its use as selection criterion for stress tolerance has been questioned.19 Thus, it is critical that tests be made before making any conclusion regarding the role of proline in stress tolerance of any specific species.20
In the previous chapter, it was shown that drought tolerance of Glaucium spp. is dependent on the internal osmoregulator content. There is no published information that addresses the mechanisms of Glaucium spp. drought tolerance. The objectives of this study were to
Lysimeter columns were used in this study which was replicated twice. All columns were placed in the plant science greenhouse at Colorado State University, Fort Collins, Co. Glaucium spp. were initiated from seeds and transplanted into potting mix, (Pro-Mix, Mycorrhizae and Biofunglcide). Fifty seedlings of each species at the 3-leaf stage were transplanted, 1 per PVS tubes (15 cm diameter and 50 cm long) containing commercial potting mix, (Pro-Mix, Mycorrhizae and Biofunglcide). The potting mix was mixed with sand 2:1 to increase pore space. The plants were maintained in the greenhouse until full establishment and recovery from transplanting. Those seedlings that survived were used as experimental units in the drought study. The experimental design was randomized complete Block (RCB). Each block contained one of the studied species with 16 tubes. Chosen seedlings had the same size and same number of leaves.
Water regimes applied included control (100% of the total evapotranspiration), as well as 75%, 50% and 25% of the total ET. ET was measured weekly. Two representative pots for each of the species were used as lysimeters and were watered with enough water and left to drain for 2h, after which the weight of each pot was recorded. Each pot was re-weighed every 24 hours. The daily changes in weight represented the daily ET for each species. Treatments were replicated four times. Seedling ET was the average of four lysimeters for each species. Treatments continued until plants reached the flowering stage. ET was updated weekly and treatments were adjusted accordingly. Over the course of the experiments data were collected weekly on plant height, leaf color, leaf area, number of flower buds, size and number of flowers, as well as quality and general attractiveness of the plant using a scale of 0 (not attractive) to 10 (optimum attractiveness). Samples were collected for TNC, RSC, and proline.
ET measurements were collected every 2 to 3 days during the four-month growth period. Five weight readings per pot were made during each measurement and the average value was used for ET calculation. ET was calculated by mass difference and expressed as mmd-1. TNC, RSC, and proline content were determined at the termination of the experiment. Shoot tissue was harvested and washed with cold distilled water to remove plant debris for carbohydrate analysis. Then, approximately 5 g of samples were freeze-dried (Genesis 25 LL Lyophilizer, Virtis, and Gardiner, NY). After freeze-drying, samples were ground with a Wiley mill, sieved thought a screen with 425 µm openings, and kept in airtight vials at–20°C. Total nonstructural carbohydrate content was measured using the method described by Chatterton et al.19 In brief, 25 mg of freeze-dried samples were transferred to 5 mL 0.1% clarase solution and incubated at 38°C for 24h. Then, 0.5 ml of hydrochloric acid (50%, v/v) was added to the incubation solution. After the solution was incubated at room temperature for 18 h, the pH value of the solution was adjusted to between 5 and 7 with 10 and 1 N NaOH. This solution was used to determine TNC content using a spectrophotometer at 515 nm wavelength (model DU640; Beckman).
To measure the free reducing sugar, 25 mg of the freeze dried, ground, and sieved sample was extracted with 10 ml of 0.1 M phosphate buffer (pH=5.4) for 24 h at room temperature. An extracted aliquot (0.2 mL) was used to determine the reducing sugar content by using the same method as was used to measure TNC.
Actual proline tissue accumulation levels were determined according to the method of Bates et al.21 as modified by Torello and Rice18 with approximately 0.5g fresh weight of tissue. Samples were ground with liquid nitrogen in a mortar. Each sample was homogenized in 10 ml of 3% aqueous sulfosalicylic acid followed by agitation for 1h prior to filtration through #2 Whatman filter paper. After filtration 2 ml of extract from each sample was reacted with 2 ml of ninhydrin reagent (1.25 mg ninhydrin in 30 mL of glacial acetic acid and 20 mL of 6 M H3PO4) and 2ml of glacial acetic acid followed by 1 h of heating at 100oC in an enclosed water bath. Samples were then quickly cooled by immersion in an ice bath and total proline was determined spectrophotometrically at 520 nm. Actual proline tissue accumulation levels were determined by subtracting mean control data from drought treatments data for all cultivars during the entire experimental period.
Data analysis
The data of the two experiments were subjected to ANOVA to test the experiment effect and the interaction between treatments and experiments. The experimental run was not significant. Therefore, data were pooled over experiments to test the effects of drought, species and their interactions using ANOVA.22 Leaf characteristics (color and area), number of flower buds, and flower characteristics (number and size) were analyzed on individual measurement dates to examine drought, and species effects over time. Means were separated by least significant difference at the 0.05 level of probability. Regression analysis was performed to determine the relationship between the measured parameters at the end of the study (dependent variables) and the water regimes (independent variable).
Leaf characteristics
Leaf color
Comparisons of leaf color among species and water regimes indicated significant differences (Table 1). At all lower water regimes, leaf color declined over time to unacceptable ratings (below 6) in G. grandiflorun and G. corniculatum. In G. flavum, and G. acutidentatum, leaf color was not adversely affected at 75% regimes (Figure 1). The effect of water stress on leaf color among all species was highly significant. The decline in leaf color was high for all species at 50% and 25% of irrigation (Figure 1). G. flavum had the highest leaf color level under all treatments. Under the control treatment, there was no difference among G. flavum, G. acutidentatum, and G. grandiflorum in leaf color (full rating of 10). They showed 100% full green leaf while G. corniculatum showed a rating of 9.5. Leaf color decreased as water regimes decreased. At the water regime of 75% of the total ET, G. flavum and G. acutidentatum did equally well and leaf color rating did not change (rating of 10). G. grandiflorum had a reduced rating of (9) while G. corniculatum rating was 8.6 at 75% ET (Figure 1). Under the lowest water regime (25% of the total ET), the leaf color of all species was adversely affected, however, only G. grandiflorum and G. corniculatum leaf color ratings were below the accepted levels (4.8 and 4.5 respectively) (Figure 1). Similarity, leaf greenness decreased under severe water stress in all almond genotypes studied by Yadollahia et al. Flexas and Medrano23,24 reported a reduction in leaf greenness in C3 plant leaves under water stress and associated that to degradation in chlorophyll content. The retention of leaves or the observation of ‘stay green’ under water stress conditions has been reported in cassava lines MH96/0686 and has correlated well with drought tolerance and improved yields in cassava.25 The decrease in relative greenness of the leaf under water stress treatment as compared to the well- watered treatment is likely due to a decrease in chlorophyll content as reported in rapeseed plants.26 There was a 38% reduction in chlorophyll content when compared to full irrigation of plants.27 Increasing water stress reduced the (Chl a) and the (Chl a:b) significantly.28 The pigment content generally decreased due to low synthesis rate and rapid degradation under water stress.23,29,30
Parameters |
Source |
||
Species (S) |
Water regimes (W) |
SXW |
|
Leaf color (0-10 scale) |
8.5** |
65.1** |
59.2* |
Leaf area (cm2) |
3.5** |
4.11** |
3.2* |
Plant height (cm) |
2.22** |
2.66** |
2.33* |
Number of buds |
29.5** |
67.0** |
20.6* |
Number of flowers |
3.2** |
6.1** |
4.9* |
Flower area (cm2) |
8.8** |
9.7** |
1.7* |
Plant quality (0-10 scale) |
8.5** |
9.6** |
6.9* |
TNC (mgg-1 dry wt) |
8800** |
711.0** |
895.0* |
RSC (mgg-1 dry wt) |
56.0** |
92.0** |
21.0* |
Proline content (µg g-1 fresh wt.) |
1270** |
1337** |
1227* |
Total ET (mm d-1) |
1.9.0** |
5.1** |
2.9* |
Table 1 Analysis of variances with mean square and treatment significance of leaf color, leaf area, plant height, number of flower buds, number of flowers, flower area, plant quality (attractiveness), total non-structure carbohydrate content (TNC), shoot reducing sugar content (RSC), proline content and total evapotranspiration in Glaucium spp
*Significant at P<0.05
** Significant at P<0.01
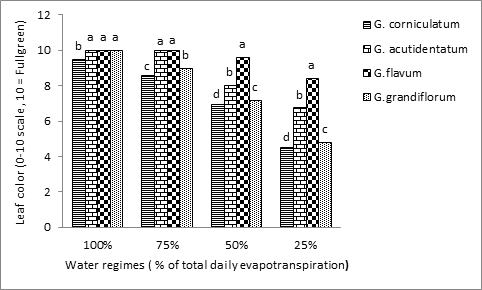
Figure 1 Effect of four different water regimes on leaf color of four Glaucium spp. Columns labeled with different letters are significantly different at P=0.05 within each water regime.
Leaf area
Analysis of variance indicated significant differences among species and among water regimes and their interactions (Table 1). Linear regression indicated a significant negative association between leaf area and water regimes (Table 2). Leaf area decreased linearly in all species with increasing drought with a sharp drop at the water regime of 25% of the total evapotranspiration. G. flavum achieved the highest leaf area at all water regimes followed by G. acutidentatum, G. grandiflorum and G. corniculatum. G. flavum acheived an average leaf area of 24.3 cm2, while G. acutidentatum achieved leaf area of 22.2 cm2 at 100% ET. G. grandiflorum was ranked third with an average leaf area of 22.0 cm2 while G. corniculatum had the lowest leaf area of 19.9 cm2 with control treatment (Figure 2). Leaf area decreased from 23 to 21.8, 18.1 and 12.5 cm2 in G. flavum; from 22.2 to 18.2, 12.5, and 10.2 cm2 in G. acutidentatum, from 22.0 to17.7, 10.6, and 7.8 cm2 in G. grandiflorum, and from 19.9 to 15.4, 10.3, and 7.0 cm2 in G. corniculatum with increased drought from the control to 75, 50 and 25% ET, respectively (Figure 2). It is logic that the leaf area followed the trend of leaf color since healthy leaves should have a greater leaf area. Although there was considerable decrease in overall leaf area in G. flavum, it appeared to be the most drought tolerant species. Water stress is one of the most common environmental factors affecting plant growth and productivity. Reduced water availability induces numerous physiological and biochemical changes in all plant organs.
Species |
Parameter |
|||||
Plant quality (0-10 scale) |
Leaf area (cm2) |
Flower area (cm2) |
||||
Regression |
R2 |
Regression |
R2 |
Regression |
R2 |
|
G. acutidentatum |
Y=4.2–0.2 X |
0.80** |
Y=102.5–2.2 X |
0.82** |
Y=210.5-6.3 X |
0.80** |
G. corniculatum |
Y=6.6–0.3 X |
0.71* |
Y=125.2–2.1 X |
0.69* |
Y=202.6-8.2 X |
0.65* |
G. flavum |
Y=7.8–0.5 X |
0.82** |
Y=116.8–2.3 X |
0.85** |
Y=113.3-8.8 X |
0.90** |
G. grandiflorum |
Y=3.9–0.3 X |
0.65* |
Y=121.2–2.5 X |
0.65* |
Y=199.0-6.8 X |
0.67* |
Table 2 Linear regression of different parameters of Glaucium spp. measured at the end of the experiment vs. water regimes of control (C), 75, 50, and 25% of the total evapotranspiration
*Significant at P<0.05
**Significant at P<0.01
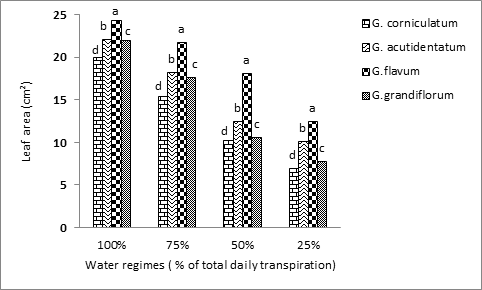
Figure 2 Effect of four different water regimes on leaf area of four Glaucium spp. Columns labeled with different letters are significantly different at P=0.05 within each water regime.
Gas exchange in leaves is limited, which in turn reduces carbon assimilation. Changes in the distribution of photo- assimilates can reduce vegetative growth31–33 as well. The reduction of leaf area is principally explained by a lower leaf unfolding rate which results in smaller leaf size.34,35 The reduction in leaf area could be an adapting mechanism to water stress. Water stress induced a significant reduction in the leaf area which is a benefit in reducing leaf transpiration.36 Similar results were found by Grant37 who studied ten strawberry cultivars under different water regimes. Furthermore, it was concluded that strawberry genotypes differed in their response to water deficiency although drought stress reduced leaf area in all of them.38 In another study, it was found that total leaf area and leaf blade area decreased with the increase in water stress in Campylotropis polyantha seedlings, while total leaf area was reduced sharply in response to progressive water stress.28 Similar results were found in eggplants,39 different almond genotypes and wheat cultivars.40,41 The common cause of the reduced vegetative growth of vegetables under water deficit conditions has been understood to be accelerated leaf senescence in eggplant.42
However, the leaf area in bell pepper was not affected by drought.43 Specific leaf area (SLA), an indicator of leaf thickness, has often been observed to be reduced under drought conditions.44 Decrease in SLA in plants under drought stress may be due to the different sensitivity of photosynthesis and leaf area expansion to soil drying. Drought stress affects leaf expansion earlier than photosynthesis.45,46 Reduction of SLA is assumed to be a way to improve water use efficiency (WUE).47–49 This is because thicker leaves usually have a higher density of chlorophyll and proteins per unit leaf area and, hence, have a greater photosynthetic capacity than thinner leaves. The mechanism, by which plant leaf area is reduced under water stress, is thought to be the reduction of cell elongation, which leads to reduction of cell size and therefore a reduction of leaf area.50
Plant height
Generally, there was a significant decrease in plant height as drought stress increased. (Table 1). G. flavum achieved an average height of 45.8 cm while G. acutidentatum averaged 40.5 cm; G. grandiflorum averaged 30.0 cm, and G. corniculatum the lowest at 27.8 cm in the control treatment (Figure 3). Plant height decreased from 45.8 to 42.0, 30.0 and 21.3 in G. flavum, from 40.5 to 33.0, 22.5, and 14.3 cm in G. acutidentatum, from 30.0 to 24.0, 13.0, and 5.3 cm in G. grandiflorum, and from 27.8 to 20.3, 9.0, and 4.5 cm in G. corniculatum as the drought increased from the control to 75, 50 and 25% of the total ET, respectively (Figure 3). Several reports have reported similar negative effects of drought on plant height.43,51–55 Previous studies indicated a significant reduction in plant height in mungbean (Vigna radiate L.),55 in Satureja hortensis,51 and in Eragrostis curvula.52 However, Alexieva43 reported no effect on pea and wheat height due to drought stress. The reduction in growth parameters such as height could be attributed to several effects such as the osmotic stress and/or ionic toxicity56 which is more harmful to plants during the succulent seedling stage in addition to the stressful effects of ion uptake.57,58 Drought stress favors the growth of roots as an adaptive mechanism rather than shoots which results in a decrease in plant height. Marcum59 reported root mass increased under stress conditions of several grasses. Also, root growth stimulation under stress conditions has been reported in stress tolerant grasses by others as well.60,61 Shahba.12 Shahba et al.13 Shahba et al.55 reported an increase in root mass of Bermuda grass cultivars and seashore paspalum cultivars under salinity and drought condition. The reduction in plant height might be due to inhibition of cell division or cell enlargement with less soil moisture availability.55,62 Rozema and Visser63 indicated that increased rooting and the associated increase in root absorbing area is an adaptive mechanism to the osmotic and nutrient deficiency stresses occurring under stress conditions which in turn results in a reduction in shoot system and plant height. Unfortunately, we were not able to measure the change in root mass in this study to support this argument in Glaucium spp.

Figure 3 Effect of four different water regimes on plant height of four Glaucium spp. Columns labeled with different letters are significantly different at P=0.05 within each water regime.
Flowering characteristics
Number of flower buds. Numbers of flower buds are varied significantly among Glaucium spp., water regimes and their interaction (Table 1). Increased water stress resulted in fewer flower buds (Figure 4). In G. flavum, as water regimes decreased from control to 75, 50 and 25 % ET, average bud number decreased by 7.8, 37.5 and 54.7% respectively. The decrease was similar in G. acutidentatum where the average number of flower buds decreased by 11, 33 and 55.6 % when drought increased from control to 75, 50 and 25%, respectively. G. grandiflorum and G. corniculatum did not produce any flower buds at an ET of 25%. This study demonstrated that drought significantly affected the production of flower buds. At the control treatment, all species produced flower buds with the highest number produced by G. flavum (32.0), followed by G. acutidentatum (22.5), G.grandiflorum (9.9) and the lowest number by G. corniculatum (8.5) (Figure 4).

Figure 4 Effect of four different water regimes on number of flower buds of four Glaucium spp. Columns labeled with different letters are significantly different at P=0.05 within each water regime.
Number of flowers. Flower number is another indicator of plant vigor. Number of flowers varied significantly (P < 0.05) among species, water regimes and their interaction (Table 1). The number of flowers declined with increased drought levels. The decline in flower number under higher drought stress was more severe and more rapid in the two less drought tolerant species (G. grandiflorum and G. corniculatum) while more moderate in G. flavum and G. acutidentatum (Figure 5). G. flavum had higher flower number under all water regimes when compared to other species (Figure 5). G. corniculatum had the lowest number of flowers at all water regimes. At the control treatment, the highest number of flowers produced was by G. flavum (26.5) followed by acutidentatum (20.5), G. grandiflorum (10) and G. corniculatum (8). Only G. flavum and G. acutidentatum produced flowers at the water regime of 25% of ET (Figure 5).

Figure 5 Effect of four different water regimes on number of number of flowers of four Glaucium spp. Columns labeled with different letters are significantly different at P=0.05 within each water regime.
Flower area. Comparisons of flower area among species and among water regimes and their interaction clearly showed significant differences (Table 1). Flower area decreased linearly with increasing drought. Regressions were strongly linear, with slope more negative with less tolerant species (Table 2). As water regime decreased, the flower area decreased. At the control treatment, flower area was the greatest in G. flavum (22.4 cm2) followed by G. acutidentatum (19.3 cm2), G. grandiflorum (10.5 cm2), and G. corniculatum had the smallest flower area (9.5 cm2) (Figure 6). The decline in flower area under substantial drought stress was more severe and more rapid in the less drought tolerant species (G. grandiflorum and G. corniculatum) while more moderate in G. flavum and G. acutidentatum (Figure 6). G. flavum had the greatest flower area under all water regimes compared to other species (Figure 6).
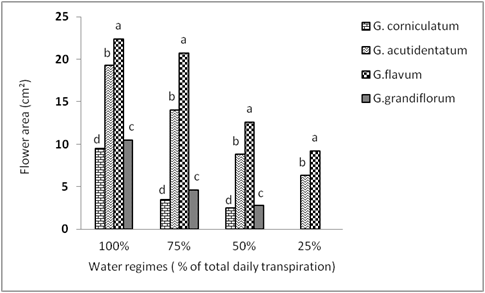
Figure 6 Effect of four different water regimes on flower area of four Glaucium spp. Columns labeled with different letters are significantly different at P=0.05 within each water regime.
Previous reports have indicated similar results in other species. Water stress affected flower induction in rice (Oryza sativa L.),64 and in satsuma mandarin Citrus unshiu Marc.65,66 Fewer flowers were often observed in cultivated satsuma mandarin under drought conditions.67 Oilseed rape was also significantly affected by water shortage during the most sensitive flowering stage.68 Koshita and Takahara65 reported negative effects on flower-bud formation in citrus because of drought as well. Southwick and Davenport66 indicated that both continuous and cyclical water-stress treatments reduced flowering of Citrus latifolia Tan. However, cotton flower buds have been shown to be relatively insensitive to water deficits.69 Flower bud induction under water stress treatments is likely due to the influence on hormonal metabolism roles. For example, plant growth regulators have been applied exogenously to elucidate the roles of plant hormones in flower-bud induction of citrus. The conclusion was that exogenously applied GA reduces the number of flowers in the following spring.70 The suppression of plant growth under drought conditions may be due to decreased availability of water that leads to the toxicity of sodium chloride.71 Also, the hydrolysis of reserved foods to produce energy necessary for biological functions and survival reduces the amount of resources available for flower formation. Drought stress imposes additional energy requirements on plant cells and less carbon is available for growth and flower primordial initiation.54,72,73 Drought effect on flower formation can be an indirect result of its effect on photosynthesis (Pn) efficiency. Pn is less sensitive to drought as compared to other growth parameters,74 but photosynthetic capacity can be reduced in the presence of great drought levels due to stomata closure, damage to photosynthetic systems by excessive energy, structural disorganization or reduction in photochemical quenching.75,76 On the other hand, Razmjoo et al.54 related the negative effects of drought on flower number to its early effect on the growth and production of a strong shoot system. Pessarakli and Touchane77 found that the reduction in biomass production due to drought stress is more obvious than the reduction in shoot lengths in bermudagrass. The decrease in plant biomass production due to drought may be attributed to low or medium water potential, specific ion toxicity, or ion imbalance that resulted from insufficient water for osmotic balance.56 In addition, elevated drought may adversely affect photosynthesis and as a result adversely affect plant biomass production through reduced accumulation of carbon products.78 The reduction in the number of flowers usually is more drastic than other growth parameters under high drought as it is a cumulative effect.54
Three contrasting faba bean genotypes (Vicia faba L.) were tested under drought stress. A reduction in the number of flowers was recorded. Saxena et al.79 concluded that the reduced flowering was a mean for maintaining stable and high seed yields under water stress. Also, reproductive development at the time of flowering is especially sensitive to drought stress.80–82 Drought stress interferes not only with flowering but also flower opening, nectar production, and turgor maintenance of floral organs as well.83 Water stress during flower induction and inflorescence development may lead to a delay in flowering (anthesis) or even complete inhibition of flowers.84,85 This confirms the differences in sensitivity to drought among different species and/or cultivars12,13,55,86–88 and even between growth stages for many plants.89 Water limitation has an impact on plant growth,90 although the exact effect may vary depending on the intensity of the water stress imposed.91 A reduction in flower size is one of the consequences of exposing plants to water stress.91 Carroll et al.92 reported that drought led to a 33% decrease in flower size relative to controls. Reduction of flower size under drought stress was recorded in populations of Clarkia unguiculata distributed along a natural moisture gradient.93 The water stress, which decreased the water potential in the soil, reduced the flower head diameter.28 The effect on flower area as related to the decrease of water availability can be explained by the decrease in the influx from the vegetative portions of the plant to the reproductive organs83 and the biochemical limitation which prevails under drought stress.94,95
Plant quality (attractiveness)
Plant quality (attractiveness) varied significantly among species and water regimes. The interaction between species and water regimes was significant as well (Table 1). Plant quality decreased linearly with increasing drought in all species. Regressions were strongly linear with larger slopes for less tolerant species (Table 2). Increasing drought decreased the attractiveness of all Glaucium spp. to different degrees (Figure 7). Under the control treatment, there was no difference between G. flavum, and G. acutidentatum since both do equally well and achieved the maximum quality (10), while there was a slightly significant difference between G. grandiflorum (9.7) and G. corniculatum (9.5) (Figure 7). The treatment of 75% ET did not have a significant effect on the quality of G. flavum or G. acutidentatum while it significantly reduced the quality of the other two species (Figure 7). All species were adversely affected at the water regimes of 50 and 25% ET, although G. flavum had less decline followed by G. acutidetutum, G. grandiflorum and G. corniculatum (Figure 7).
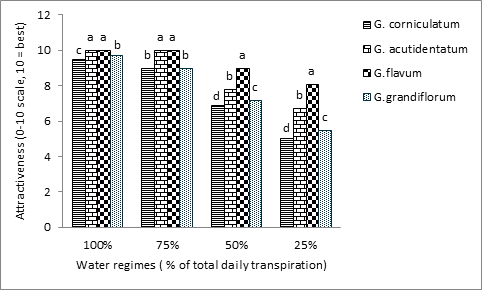
Figure 7 Effect of four different water regimes on the attractiveness of four Glaucium spp. Columns labeled with different letters are significantly different at P=0.05 within each water regime.
Plants express various responses to drought and develop a wide range of tolerance strategies that affect both morphological and physiological traits.96 These responses may be reflected in plant leaf greenness, leaf size, plant height and flowering quality. Water stress has been shown to significantly reduce plant size.97 Studies have also shown that drought stress can affect the growth of plant organs differently98 which may result in the alteration of morphology.99 Putievsky et al.100 reported that water stress had a negative impact on green tissue yield of geranium. Drought caused reduction in all growth parameters of Matricaria chamomile.54 Furthermore, a study by Flexas and Medrano24 showed that moisture deficiency affects various physiological and metabolic responses such as stomatal closure, decline in growth rate and photosynthesis. Also, Baher et al.51 showed that greater soil water stress decreased plant height and total fresh and dry weight of Satureja hortensis. Colom and Vazzana52 showed that the number of branches per plant and total plant dry weight was negatively affected by water stress in Eragrostis curvula. The range of drought in which the plant is able to survive varies according to the species.101 The ability to limit Na+ transport into the shoots, and to reduce the Na+ accumulation in the rapidly growing shoot tissues, is critically important for maintenance of high growth rates and protection of the metabolic process in elongating cells from the toxic effects of Na+54 which is a process that requires sufficient water in plant cells. The quality of lilies (plant height, flower bud length and flower diameter) decreased as water relations changed because of osmotic imbalances.73 Also, drought may directly or indirectly inhibit cell division and enlargement and finally the growth of the whole plant.
Some above ground visible morphological symptoms of plants are marginal yellowing/browning of foliage, premature fall of leaves, twig and branch die back, loss of vigor and stunted growth. Several previous studies have found similar results to our findings. Drought caused a decline in the quality of bermudagrass cultivars13 and seashore paspalum cultivars.102 In addition, elevated drought may adversely affect photosynthesis and as a result adversely affect plant biomass production through reduced accumulation of carbon products.78 The reduction in the number of flowers usually is more drastic than other growth parameters under high drought as it is a cumulative effect.54 Fewer flowers and reduced size of flowers adversely affect the attractiveness of landscape plants.
Water use efficiency
Drought avoidance is an important drought resistance strategy. Drought avoidance can be achieved through the reduction in water use or water loss through the canopy and increasing water uptake of roots from deeper soils. ET is a measure of water use efficiency and is an indicator of plant vigor. ET varied significantly (P<0.05) among species under different water regimes, among water regimes and their interaction (Table 1), (Table 2) (Table 3). Regression analysis indicated a significant negative linear relationship between water regimes and ET rates (Table 3). ET rate declined with the reduction in irrigation water. The decline in ET rate under lower water regimes was more severe and more rapid (Table 3). G. flavum showed lower ET rates under all water regimes when compared to G. acutidentatum, G. grandiflorum and G. corniculatum. G. corniculatum had the highest ET rates at all water regimes (Figure 3). Transpiration efficiency (TE) has been identified as one of the important physiological traits for improving drought adaptation of plants. The variation in TE is associated with variation in photosynthetic capacity per unit leaf area because thicker leaves usually have a higher density of chlorophyll per unit leaf area and hence have a greater photosynthetic capacity when compared with thinner leaves. Leaf thickness may also affect plant quality. G. flavum showed greater TE since it was able to maintain its ET at lower rates while maintaining higher attractiveness when compared with G. acutidentatum which was next in TE with G. grandiflorum and G. corniculatum which had the lowest TE (Table 3). Many species have shown considerable interspecific diversity for various environmental stresses, including drought.10,103 Kim and Beard104 found that species/cultivar differences in ET rates under non-limiting soil moisture conditions were associated with canopy resistance and total leaf area. High canopy resistance and/or a low leaf area resulted in lower ET. Arunyanark et al.105 reported a reduction in transpiration rate because of drought while the transpiration efficiency, as indicated by total dry matter production, was increased in peanut (Arachis hypogaea L.).
Species |
ET rate (mmd-1) |
Regression |
R2 |
|||
Water regimes (% of total ET) |
||||||
C |
75 |
50 |
25 |
|||
G. acutidentatum |
4.0c† |
3.4c |
2.4c |
1.7c |
Y=22.0–0.9 X |
0.79** |
G. corniculatum |
5.2a |
4.5a |
3.0a |
2.5a |
Y=12.6 –1.2 X |
0.64* |
G. flavum |
4.0c |
3.3c |
2.2c |
1.2d |
Y=11.8–1.6 X |
0.80** |
G. grandiflorum |
4.4b |
3.9b |
2.7b |
2.2b |
Y=10.7– 0.8 X |
0.72* |
Table 3 Effect of different water regimes on daily ET (mmd-1) of Glaucium spp. linear regression of different ET rates vs. water regimes of control (C), 75, 50, and 25% of the total evapotranspiration
† Values followed by the same letters within a column for each cultivar are not significantly different (P=0.05) based on a Fisher’s LSD test
*Significant at P<0.05
**Significant at P<0.01
Osmotic adjustment
Osmotic adjustment facilitates water uptake and limits water loss from cells. Thus, tissues may sustain metabolic and physiological functions under drought stress in addition to the stability of cell membrane. Tested osmotic adjustment parameters included shoot total nonstructural carbohydrates, total reducing sugar content and shoot proline content.
Shoot total nonstructural carbohydrates and total reducing sugar content
Shoot TNC varied significantly among species, water regimes and their interaction (Table 1). Increasing drought decreased shoot TNC of Glaucium spp. (Table 4). Regression analysis indicated a significant negative linear relationship between water regimes and TNC content (Table 4). In G. flavum, as water regimes increased from control to 75, 50 and 25 % of the total ET, average TNC decreased by 15.1, 30.3 and 48.0% while the average TNC decrease in G. acutidentatum shoots was 21.6, 40.1, and 53.7%. The decrease in G. grandiflorum was 21.4, 42.7 and 54.8% while the decrease in G. corniculatum was 27.0, 53.7 and 59.4%, respectively. A decline in TNC was most likely due to the decline in photosynthesis because of stomatal closure as a water saving mechanism. Shoot RSC varied significantly among species, water regimes and their interactions (Table 1). RSC response to different drought treatments followed a different trend than TNC (Table 5). Reducing sugars in plants mainly consists of glucose and fructose.101,102 While nonstructural carbohydrates are energy reserves in plants, soluble reducing sugars are thought to play an important role in drought, salinity and freezing tolerance as osmoregulators and as protectants as they prevent cell desiccation.106 Regression analysis indicated a significant positive association between drought and RSC content in all species at all water regimes (Table 5). As water regimes increased from control to 75, 50 and 25% ET, average RSC increased by 40.7, 101.8 and 166.5 % in G. flavum and by 17.4, 40.0 and 103.4% in G. acutidentatum. The increase was 122.2, 39.6, and 90.6% in G. grandiflorum and 4.4, 26.5, and 62.5% in G. corniculatum, respectively. Carbon reduction could be related to the drought resistance mechanisms that are energy dependent. The results suggested that carbohydrate availability was a limiting factor for shoot growth under high drought stress. Shahba13 found an increase in RSC and a decrease in TNC with drought increase in bermudagrass species (Tifgreen, Tifdwarf and Tifway) and seashore paspalum cultivars.12,13
Species |
TNC (mgg-1 dry wt) |
Regression |
R2 |
|||
Water regimes (%) |
||||||
C |
75 |
50 |
25 |
|||
G. acutidentatum |
120.5b† |
94.5b |
72.2b |
55.8b |
Y=122.5–2.1X |
0.82** |
G. corniculatum |
98.3d |
71.8d |
45.5d |
39.9d |
Y=108.6–2.0X |
0.79* |
G. flavum |
126.6a |
107.5a |
88.2a |
65.8a |
Y=107.2–1.9X |
0.86** |
G. grandiflorum |
103.8c |
81.6c |
59.5c |
46.9c |
Y=115.3–1.8X |
0.76* |
Table 4 Total nonstructural carbohydrates (TNC) in shoots of Glaucium spp. measured at the end of the experiment vs. water regimes of control (C), 75, 50, and 25% ET
†Values followed by the same letters within a column for each cultivar are not significantly different (P=0.05) based on a Fisher’s LSD test
*Significant at P<0.05
**Significant at P<0.01
Species |
RSC (mg g-1 dry wt) |
Regression |
R2 |
|||
Water regimes (%) |
||||||
C |
75 |
50 |
25 |
|||
G. acutidentatum |
17.8 |
20.9b† |
24.2b |
36.2b |
Y=20.5+0.14 X |
0.78** |
G. corniculatum |
13.6 |
14.2d |
17.2d |
22.1d |
Y=15.2+0.13 X |
0.70* |
G. flavum |
16.7 |
23.5a |
33.7a |
44.5a |
Y=10.9+0.25 X |
0.88** |
G. grandiflorum |
14.9 |
18.2c |
20.8cb |
28.4c |
Y=14.5+0.14 X |
0.75* |
Table 5 Total reducing sugar content (RSC) in shoots of Glaucium spp. measured at the end of the experiment vs. water regimes of control (C), 75, 50, and 25% ET
† Values followed by the same letters within a column for each cultivar are not significantly different (P=0.05) based on a Fisher’s LSD test
*Significant at P<0.05
**Significant at P,0.01
Soluble carbohydrates may interact with membrane phospholipids and proteins to stabilize their structures and prevent desiccation under drought stress.106 TNC serves as the resource for the increased RSC under drought conditions. The balance between carbohydrate production and consumption impacts the ability of plants to cope with stresses.9,10–13
Shoot proline content
Shoot proline content varied significantly among species, water regimes and their interaction (Table 1). Increasing drought increased shoot proline content of Glaucium species. The increase in proline content was more obvious with increasing drought (Table 6). As water regimes decreased from control to 75, 50 and 25% average proline content in shoots increased by 186, 325, and 472% in G. flavum, 163, 303 and 517% in G. acutidentatum, 160, 280 and 418% in G. grandiflorum and 80, 190, and 340% in G. corniculatum, respectively. Regression analysis indicated a significant positive association between drought and proline content in all species (Table 6). Although the role of proline accumulation in drought tolerance is well documented in this study, it has been questioned by others.19 Our results suggest a positive role for proline in Glaucium species drought tolerance. A positive effect of proline accumulation in drought tolerance was also reported in seashore paspalum cultivars.55 Accumulation of proline in plant tissues in response to drought stress has been attributed to enzyme stabilization and/or osmoregulation.15,16 It could act as a sink for carbon and nitrogen for stress recovery and may buffer cellular redox potential under drought stress.14 Maggio et al.107 suggested that proline may act as a signaling/regulatory molecule able to activate multiple responses that participate in the adaptation process to environmental stresses. Little is known of metabolic factors controlling root survival in drying soils and the proteins or genes associated with the accumulation of osmolytes.108 The accumulation of solutes in leaves, such as soluble sugars, inorganic ions, and proline has been associated with osmotic adjustment and increased drought tolerance in Kentucky bluegrass,109 tall fescue,110 perennial ryegrass,111 and zoysiagrass.112 Osmotic adjustment has also been observed in roots of crops which contribute to the maintenance of root turgor and elongation in dry soils.113 A positive correlation between the capacity of osmotic adjustment and recovery from prolonged drought has been reported in several species, where species with the greatest osmotic adjustment regrew faster after watering.114 Any cultural practice that promotes accumulation of osmotic solutes during drought stress should be helpful in landscape plants for rapid recovery from that stress. On the basis of best results relative to categories for leaf characteristics, plant height, flowering characteristics, overall plant quality (attractiveness), water use efficiency, TNC, RSC, and proline, G. flavum was found to have greater drought tolerance when compared to G. acutidentatum, G. grandiflorum and G. corniculatum. In summary, as drought increased, Glaucium spp. exhibited reduction in leaf characteristics, plant height, flowering characteristics, overall plant quality (attractiveness), TNC, and ET rate, and increased shoot total reducing sugars and proline content. G. flavum showed greater drought tolerance at all water regimes compared to the other tested species. Proline accumulation could add to the drought tolerance through osmoregulation or by acting as carbon and nitrogen sink for stress recovery.
Species |
Proline content (µg g-1 fresh wt) |
Regression |
R2 |
|||
Water regimes (%) |
||||||
C |
75 |
50 |
25 |
|||
G. acutidentatum |
243.0 |
639.0b† |
980.0b |
1499.0b |
Y=218.3+22.9 X |
0.82** |
G. corniculatum |
226.9 |
408.0d |
659.0d |
998.0d |
Y=144.5+14.5X |
0.72* |
G. flavum |
281.5 |
805.0a |
1195.0a |
1610.0a |
Y=139.6+11.4 X |
0.90** |
G. grandiflorum |
223.2 |
580.0c |
849.0c |
1155.0c |
Y=172.2+18.4 X |
0.74* |
Table 6 Proline content in shoots of Glaucium spp. measured at the end of the experiment vs. water regimes of control (C), 75, 50, and 25% ET
†Values followed by the same letters within a column for each cultivar are not significantly different (P=0.05) based on a Fisher’s LSD test
*Significant at P<0.05
**Significant at P<0.01
None.
Authors declare that there is no conflict of interest.

©2019 Getlawi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.