eISSN: 2576-4462


Research Article Volume 8 Issue 1
1Estación Territorial de Investigaciones de la Caña de Azúcar Centro Villa Clara (ETICA Centro-Villa Clara). Instituto de Investigaciones de la Caña de Azúcar (INICA). Cuba
2Instituto de Investigaciones de la Caña de Azúcar (INICA), Cuba
3Grupo de Extensión y Servicios Ciego de Ávila (GESA-Ciego de Ávila) Instituto de Investigaciones de la Caña de Azúcar (INICA), Cuba
4Instituto de Tecnología Agroindustrial del Noroeste Argentino (ITANOA) y Estación Experimental Agroindustrial Obispo Colombres (EEAOC), Tucumán, Argentina
5Faculty of Science & Technology, University of Belize, Belize, Central America
Correspondence: Aydiloide Bernal Villegas, Estación Territorial de Investigaciones de la Caña de Azúcar Centro Villa Clara (ETICA Centro-Villa Clara). Instituto de Investigaciones de la Caña de Azúcar (INICA). Autopista Nacional km 246, Ranchuelo,Villa Clara, Cuba
Received: January 15, 2024 | Published: January 31, 2024
Citation: Villegas AB, Luz la O M, Acevedo R, et al. Production of phenolic compounds from in vitro shoots of sugarcane (Saccharum spp.) in temporary immersion bioreactors. Horticult Int J. 2024;8(1):1-6. DOI: 10.15406/hij.2024.08.00294
An alternative way to obtain molecules of high-value from plant origin for the agricultural and pharmaceutical industries is via the production of secondary metabolites from in vitro systems. Among these molecules are phenolic compounds that are important in plant defense system against biotic and abiotic factors. The objective of this work was to establish the culture conditions for the production and scaling up of phenolic compounds from sugarcane shoots produced in Temporary Immersion Bioreactors (TIBs). Two different sucrose concentrations and three inoculum densities of shoots per flask were tested for two sugarcane cultivars C86-56 and C1015-73. These cultivars were micropropagated in TIBs without ascorbic acid for phenolic compound production during 25 days, and were measured every five days. The best established conditions were evaluated on a pilot scale with ten TIBs for three sugarcane cultivars (C86-56, C1051-73 and C87-51). Although phenolic compounds were produced under all conditions evaluated at the experimental level, the most important production was achieved by using 15 shoots per vessel with 20 g.L-1 of sucrose in the absence of ascorbic acid after 25 days of culture. Cultivar C1051-73 had a higher multiplication coefficient independently of the treatments; this effect was not only significant for the phenolic compound production, but also for biomass increases. With respect to the pilot experiment, significant differences were detected for phenolic compound production among the three cultivars with C1051-73 showing the highest yield (23.05 mg.L-1). The results revealed that TIBs are a useful method to produce phenolic compounds with optimum yields.
Keywords: in vitro culture, phenols, sugarcane, temporary immersion bioreactors
Sugarcane (Saccharum spp.) is one of the most important crops worldwide. It is distributed in 100 countries over an area of 26 million hectares mainly in the tropical and subtropical regions. It ranks 12th out of a total of 161 species of agro-industrial interest.1 In Cuba, it is distributed throughout the national territory, with an area of 642,243.3 ha planted.2
Biotechnology in this plant species is an important tool for genetic improvement and mass propagation. The first research related to the culture of cells and tissues in sugarcane began in 1961 in Hawaii, USA.3,4
The semi-automation of the process is a more efficient alternative to increase the multiplication coefficients, the quality of the shoots and reduction of costs.5-7 Temporary Immersion Systems (TIS) were developed several decades ago for the in vitro culture of shoots and roots.8 Within these, the Temporary Immersion Bioreactors (TIBs) are used for the in vitro propagation of sugarcane worldwide.9,10
These provide a more favorable environment because they offer the possibility of controlling the chemical and physical variables within the culture vessels.7 However, the presence of phenols and their oxidation causes tissue damage in vitro that can eventually lead to cell death, which is indicated by the darkening of the culture medium.11,12 In different plant species, a direct relationship was found between the genotype, source of the explant and composition of the culture medium.13
At present, the objective of the pharmaceutical and food industries is the development of technologies, where it is possible to obtain high yields of secondary metabolites with the use of in vitro culture.14 In several plant species these systems are used in the production of biomass for this purpose.15-18
Studies of phenolic compounds are still in its initial stages due to the complexity of their production.19 These are classified according to their biosynthetic routes as terpenes, glycosides, alkaloids and phenolic compounds.20,21 These organic compounds are important in the defense system of plants against biotic and abiotic external aggressors.18,22-24
To date, the culture conditions for the production of phenolic compounds from in vitro shoots of sugarcane propagated in TIBs have not been studied. Lorenzo et al.9 stated that these phenols do not affect growth and development of the plants, so their production would add value to this methodology.
The objective of this research is to establish the culture conditions for the production and scaling up of these compounds from in vitro shoots of sugarcane in Temporary Immersion Bioreactors.
The present research was developed in the Biofactory located in the Sugarcane Experimental Station (ETICA Centro Villa Clara), belonging to the Research Institute of Sugar Cane (INICA). The quantification of the phenolic compounds was carried out at the Agro-Industrial Experimental Station "Obispo Colombres" (EEAOC), San Miguel de Tucumán, Argentina.
General Procedures
In all the stages of work under in vitro conditions, the plant material was taken from shoots of sugarcane plants obtained through shoot tip culture, according to the methodology proposed by Jiménez.25 The cultivars C1051-73, C86-56 and C87-51 were used as plant material.
Culture media
In the multiplication phase for temporary immersion, liquid culture medium composed of 100% MS salts,26 1 mgL-1 thiamine; 100 mg.L-1 of myo-inositol; 4 mgL-1 of 6-benzyl aminopurine; 30 gL-1 of sucrose and 50 mgL-1 of ascorbic acid were used. The pH was adjusted to 5.6 with NaOH (1.0 N) and HCl (1.0 N) prior to sterilization.
In vitro culture conditions
The TIBs were placed in the growth chamber with sunlight at a temperature of 27 ± 2ºC. The photoperiod was 13 hrs. light with photosynthetic photon flux density (PPFD) between 17.1 and 64.6 μmol m-2s-1, measured with an Extech 401025 Luxometer (Extech Instruments, USA).
The immersion frequency of in vitro shoots with the culture media during the entire experimental work was every 3 h for 5 min according to Lorenzo et al.9 In all the experiments, the inoculum density of 20 shoots per bioreactor and the culture medium described initially was taken as control.
Determination of sucrose concentration, inoculum density and genotype during the production of phenolic compounds.
The purpose of this experiment was to determine the appropriate conditions for the production of phenolic compounds in TIBs. For which two concentrations of sucrose 20 and 30 gL-1 were used, three inoculum densities (10, 15 and 20 in vitro shoots) per culture vessel in the absence of ascorbic acid for the cultivars C86-56 and C1015-73. As a control, 30 gL-1 of sucrose and 20 shoots per vessel were used in vitro with ascorbic acid (normal culture conditions for propagation in vitro in TIBs in sugarcane). Three TIBs were used per treatment and repeated three times.
After 21 days, the darkening was determined according to the methodology described by Campos-Vargas et al.27 and the absorbance was read at 320 nm in a UV-VIS spectrophotometer (PharmaSpec UV-1700, Shimadzu, Kyoto, Japan). The height (cm) of 30 in vitro shoots from the base to the insertion of the first fully expanded leaf was measured and the multiplication coefficient was determined by dividing the total number of final in vitro shoots over the initial amount.
For the statistical analysis, a factorial analysis of variance (ANOVA) was applied and the difference between the means of the treatments was determined by Fisher's Least Significant Difference (LSD) test for p≤0.05.
Determination of the culture time for the production of phenolic compounds
To determine the culture time for the production of phenolic compounds, this study was repeated with the best combinations obtained from the previous experiment and a control with ascorbic acid was included. As a repetition, three TIBs were used per treatment.
The coloration of the culture medium and shoots in vitro was estimated using the hexadecimal color code.28 For the statistical analysis, an ANOVA was applied and the difference between the means of the groups was determined by Fisher's Least Significant Difference (LSD) test for p≤0.05.
Quantification of total phenols produced in TIBs on a pilot scale
The objective of this experiment was to quantify the production of phenolic compounds on a pilot scale with 10 TIBs of the sugarcane cultivars (C86-56, C1051-73 and C87-51) after 25 days of culture that were in the productive phase of the Biofactory, during in vitro propagation within the commercial production scheme.
The variables analyzed were measurement of the absorbance at 320 nm to determine the presence of phenolic compounds, the multiplication coefficient of 30 in vitro shoots by TIB and the content of total phenols (mgL-1) was determined through the Folin-Ciocalteu method.29
For the statistical analysis, an analysis of variance (ANOVA) of simple classification was applied and the difference between the means of the groups was determined by the Tukey test for p≤0.05.
Determination of sucrose concentration, inoculum density and genotype during the production of phenolic compounds.
It was determined that there are no significant differences between cultivars, sucrose concentrations and inoculum density that were evaluated to be able to select the best combination, as well as in the interactions between these factors.
The shoots in vitro of sugarcane of the cultivars C86-56 and C1051-73 achieved excretion of phenolic compounds during their multiplication in the TIB in the absence of ascorbic acid, with significant differences with respect to the control (Table 1). In both cultivars with 20 g.L-1 of sucrose and an inoculum density of 15 shoots, the highest yields of these compounds and heights were obtained. The latter guarantees a better survival rate in later stages within the process of in vitro propagation.
The cultivar C1051-73 had a higher multiplication coefficient independently of the treatments. This is very important because in addition to producing more phenolic compounds, the number of plants increased.
Different letters in the same column represent significant differences according to the Fisher's Least Significant Difference test for p <0.05. For the variables height and multiplication coefficient (n = 90) Mean ± 0.023 and for the OD (n = 9) Mean ± 0.051.
With respect to the height for both cultivars, the highest values were obtained when 20 g.L-1 of sucrose (always above 7.0 cm) was used, with significant differences compared to the rest of the treatments. Although this variable should be considered as a trait of the cultivar, the results showed that the secondary metabolites produced did not affect the growth of the shoots in vitro (Table 1).
|
Cultivar |
Sucrose g.L-1 |
Density (shoots L-1) |
OD 320 nm |
OD 437 nm |
Height (cm) |
Multiplication coefficient |
|||
|
|
|
10 |
0,382 c |
0,488d |
7,33 a |
90,5 b |
|
||
|
|
20 |
15 |
0,508 a |
0,620 b |
7,20 a |
91,3 b |
|
||
|
|
20 |
0,485 a |
0,614 b |
6,98 a |
88,3 b |
|
|||
|
C86-56 |
10 |
0,360 c |
0,506 d |
6,45 b |
89,5 b |
|
|||
|
|
30 |
15 |
0,423 b |
0,510 d |
6,30 b |
87,8 b |
|
||
|
|
20 |
0,442 b |
0,544 c |
6,12 c |
86,0 b |
|
|||
|
(Control) |
30 |
20 |
0,046 e |
0,420 e |
5,92 c |
89,4 b |
|
||
|
|
10 |
0,417 b |
0,495 d |
7,38 a |
120,3 a |
|
|||
|
|
20 |
15 |
0,517 a |
0,679 a |
7,18 a |
121,6 a |
|
||
|
|
20 |
0,494 a |
0,632 b |
6,40 b |
116,1 a |
|
|||
|
C1051-73 |
10 |
0,381 c |
0,483 d |
4,10 d |
122,8 a |
|
|||
|
|
30 |
15 |
0,406 c |
0,491 d |
3,95 d |
113,0 a |
|
||
|
|
20 |
0,424 b |
0,526 c |
3,56 e |
114,1 a |
|
|||
|
(Control) |
30 |
20 |
0,039 e |
0,350 e |
3,70 e |
115,2 a |
|
||
Table 1 Production of phenolic compounds from the multiplication of shoots in vitro of the sugarcane cultivars (C86-56 and C1051-73) (Saccharum spp.) in Temporary Immersion Bioreactors at 21 days of culture with different sucrose concentrations and inoculum densities.
In the case of the cultivars, it was determined that C1051-53 had an integral production of phenolic compounds. In this regard, the influence of the genotype on the different processes that occur during in vitro culture is known.
With respect to the inoculum density used by each cultivar, it was found that when 15 in vitro shoots were used per culture vessel, higher production of secondary metabolites was obtained in both cases, hence making the use of the culture vessel and the culture medium more efficient. According to Dias et al.,19 plants naturally produce phenolic compounds; however, when placed in vitro conditions it is necessary to manage different culture conditions and components of the culture medium for its production.
The use of 20 g.L-1 of sucrose in the culture media in the TIB in this research was able to stimulate the production of phenolic compounds, which is supported by what was proposed by Wind et al.30 with respect to the inversely proportional relationship between the said carbon source and the activity of the enzymes involved in the phenylpropanoid pathway (superoxide dismutase and peroxidase).
In research carried out by Baque et al.,31 it was noted that the initial concentration of sucrose may affect the production of phenolic compounds in adventitious root cultures of Morinda citrifolia L. in TIB. This varies with the species and between different cultivars. The best results were achieved with 10 gL-1 of sugar in the culture medium. Also, Fazal et al.32 with 20 g.L-1 of sucrose in cell suspensions of Prunella vulgaris L. achieved the same results as those of the present research work. Regarding the initial inoculum density, Lorenzo et al.9 showed that there is a close relationship between the production of phenolic compounds and the formation of shoots in vitro.
Determination of the culture time for the production of phenolic compounds
In the shoots of the sugarcane cultivars C86-56 and C1051-73, the production of phenolic compounds was significantly increased in the culture medium during the 25 days in the multiplication stage in the TIB with respect to the control with ascorbic acid (Figure 1). This indicates the active participation of the phenylpropaniods route.
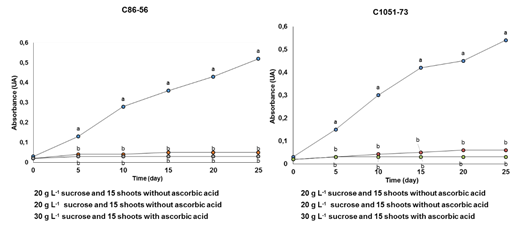
Figure 1 Effect of the culture time on the production of phenolic compounds (wavelength at 320 nm) during the multiplication of in vitro shoots in the Temporary Immersion Bioreactors (A) cultivar C86-56 and (B) cultivar C1051-73.
Lines with different letters differ significantly according to Tukey's test for p≤ 0.05 (n = 20), (A) Averages = 0.145 ± 0.054 Standard Error (SE) and (B) Averages = 0.154 ± 0.011 SE
The basic concentrations of phenolic compounds in the culture media with ascorbic acid could be due to the wounds caused by the manipulation of the explants during subculturing of the shoots in vitro in the TIB. The highest absorbance values were reached after 25 days of culture with significant differences with the treatments where ascorbic acid was present as an antioxidant.
In both cultivars the treatment without ascorbic acid and 15 in vitro shoots as the inoculum density showed the darkening of the culture medium which turned a golden wire color (code DAA520) after 15 days. Thereafter it turned to a brown mount color (code 993300)28 after 25 days of culture. This was due to the higher production of phenolic compounds, which was corroborated with the absorbance readings at 320 nm.
The in vitro shoots presented a forest green color (code 228B22). The culture media with ascorbic acid and 15 shoots per culture vessel maintained the pale golden wire color (code FFFF99) and its transparency throughout the multiplication phase in the TIBs. The shoots in vitro presented a pale green coloration (code 99FF99)28 up until 25 days of culture.
These results indicate that the concentration of the phenolic compounds increased with the intermittent contact of the sugarcane shoots with the culture medium. In addition, the duration of this phase was reduced by five days with respect to the work taken as reference, where it was reported by Lorenzo et al.9 that it takes 30 days to get the plant material ready for the next subculture.
Temporary immersion systems are an efficient tool to improve the in vitro, multiplication of shoots and plants which was referenced by several authors such as Escalona et al.,8 Etienne et al.,33 Watt.7 However, other researchers reported these systems for obtaining secondary metabolites.15,17,34
Quantification of total phenols produced in TIBs on a pilot scale
The highest absorbance value of the crude extracts was reached during the multiplication of in vitro shoots in the TIBs after 25 days of culture in the cultivar C1051-73 (0.72 AU), with significant differences with the other two cultivars C87-51 and C86 -56. The latter cultivar had the lowest darkening potential with 0.47 AU (Figure 2). These results were corroborated with the scaling up in the productive process of the biofactory on in vitro culture conditions that were established experimentally.
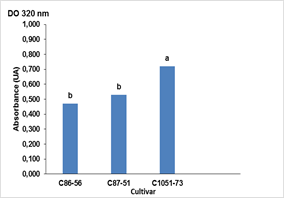
Figure 2 Production of phenolic compounds in three cultivars (C86-56, C87-51 and C1051-73) of sugarcane (Saccharum spp.) cultured in Temporary Immersion Bioreactors (TIBs) without ascorbic acid at 25 days of culture in the productive process of the Biofactory.
Bars with different letters differ significantly according to Tukey's test for p≤0.05 (n = 30 TIBs) Averages ± Standard Error (SE) 0.56 ± 0.083 for absorbance readings at 320 nm
The cultivar C1051-73 had the highest excretion of phenolic compounds with 23.05 mg.L-1, with significant differences with the other two cultivars multiplied in the TIBs and the control treatment (Figure 3). This represented a total of 345.7 mg in the 10 large culture vessels, which is an acceptable amount for further separation and purification. There was a direct relationship between the absorbance at wavelength 320 nm of the crude extracts and the concentration of phenols.

Figure 3 Content of total phenols by the Folin-Ciocalteu technique in crude extracts obtained from in vitro shoot excretion in three cultivars of sugarcane (Saccharum spp.) multiplied in the Temporary Immersion Bioreactors (TIBs) at 25 days of culture.
Bars with different letters differ significantly according to the Tukey test for p≤ 0.05 (n = 30 TIBs) Averages ± Standard Error (SE) 14.52 ± 2.81
The concentrations of total phenols obtained in the present work with the in vitro culture conditions established for sugarcane (C1051-73) in the TIBs, was superior to the same cultivar reported by Lorenzo et al.9 with values of 15.0 mg.L-1 during 30 days of culture. This confirms the importance of establishing the most appropriate in vitro culture conditions to achieve the proposed objective.
Also Duarte-Almeida et al.35 stated that in the sap obtained from the stem of the Brazilian sugarcane cultivar SP 801842, 160 mgL-1 of total phenolic compounds was obtained using the Folin-Ciocalteu method. Cultivar C86-56 produced the lowest concentrations of total phenols, which corroborated the results of Abbas et al.36 who found different contents of phenolic compounds in 12 sugarcane cultivars.
The accumulation of phenolic compounds during the multiplication of shoots in vitro in stationary liquid culture media of St. John's wort (Hypericum perforatum L. St. Johns Wort) and in schisandra (Schisandra chinensis Turcz.),37,38 was observed without affecting their development. This confirms that phenols do not always cause tissue damage under these growing conditions, which can be managed to increase their production.
According to Compton and Preece,39 secondary metabolites are not necessarily harmful to crops and showed that catechol and phloroglucinol promoted the formation of blackberry (Rubus spp) shoots. All of the above highlights the importance of the standardization of culture conditions for the production of these compounds from in vitro sugarcane shoots in the TIBs.
To date there is little information on the culture conditions for obtaining phenolic compounds in sugarcane. It was determined that with 20 gL-1 of sucrose, 15 in vitro shoots as the initial inoculum density and a culture medium without antioxidant, the highest production of phenolic compounds was possible after 25 days of culture.
During the development of this experiment, it was possible to achieve the production of 23.05 mgL-1 of phenolic compounds in the cultivar C1051-73 during the multiplication in TIBs. This methodology allows scaling up of these secondary metabolites, which can be used in the cosmetic, pharmaceutical and agricultural industries.40
None.
There were no conflicts of interest.

©2024 Villegas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.