eISSN: 2576-4462


Research Article Volume 1 Issue 2
GB Pant National Institute of Himalayan Environment and Sustainable Development, India
Correspondence: Aseesh Pandey, GB Pant National Institute of Himalayan Environment and Sustainable Development, Sikkim Unit, Pangthang, Gangtok-737101 (Sikkim), India, Tel 919458940052
Received: September 14, 2017 | Published: October 12, 2017
Citation: Bahukhandi A, Pandey A, Sekar KC, et al. Polyphenolics, nutrients and antioxidant activity of Gaultheria trichophylla royle: a high value wild edible plant of trans himalaya. Horticult Int J. 2017;1(2):39-43. DOI: 10.15406/hij.2017.01.00007
Gaultheria trichophylla Royle (Family-Ericaceae), a highly valued wild edible plant of Trans Himalaya, is used for the treatment of various ailments in traditional medicinal systems. The present study focuses on analyzing biochemical attributes of G. trichophylla fruits collected from different sampling sites located at different altitudes in the Trans Himalayan zone of Uttarakhand, India. The primary phytochemical analysis of polyphenolics, nutrients and antioxidant activity showed a strong positive correlation (p<0.05) with altitude. Additionally, phenolics exhibited significant (p<0.05) relationship with antioxidant activity and nutrient contents. Overall, G. trichophylla was found a potential source of natural antioxidants with high nutritious potential. Planning of large scale propagation of this species is recommended for better production through plantation of this species under rural development programs across the Trans Himalayan villages.
Keywords: gaultheria trichophylla, polyphenolics, antioxidant activity, wild edible, trans himalaya
GAE, gallic acid equivalent; TAE, tannic acid equivalent; QE, quercetin equivalent; AAE, ascorbic acid equivalent; CN, cyanidin 3-glucoside; ABTS, 2, 2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid); DPPH, 2, 2-diphenyl-1-picryhydrazyl; FRAP, ferric reducing antioxidant power; TPTZ, 2,4, 6-tri-2-pyridyl-1,3,5-triazin; FW, fresh weight
In spite of overwhelming growth in allopathic medicines during 20th century, plants still remain one of the major sources of natural antioxidants. In human body, Reactive Oxygen Species (ROS) are formed either as an essential mediator in vital processes including neurotransmission and inflammatory reactions or as a by-product.1 The overproduction of free radicals, and imbalance of oxidants and antioxidants constituents in the bodies, can cause oxidative damage to biomolecules i.e. lipids, proteins, etc. and create several type of syndromes / non-functions such as cardiovascular dysfunctions, atherosclerosis, inflammation, carcinogenesis, drug toxicity, aging, reperfusion injury and neurodegenerative diseases.2 Among others, the wild edible plants, found in natural habitats, are potent source of natural secondary metabolites, which protect plants from different type of stress and ultra violet radiations.3 These secondary metabolites maintain the balance of oxidant and antioxidant compound in human body.4 Indian Himalayan Region (IHR) is one amongst the biodiversity rich regions in the world and over 675 wild edible plants have been documented from this region.5 The diversity of wild edible plants in IHR are traditionally been known, and these plants have been consumed as nutritious food, source of medicine and remained part of human health from time immemorial.6 Studies have suggested that fruits are rich source of phenolics, flavonoids, tannins, anthocyanins, vitamins and antioxidants, and their regular consumption associated with reducing the risk of cancer, cardiovascular syndrome, aging and neurodegenerative diseases.7,8
Gaultheria trichophylla Royle (family-Ericaceae), a high value wild edible plant, is decumbent, mat-forming aromatic shrub in higher altitude zone around 3200-5300 m asl. Species is widely distributed across the Trans Himalayan zones of India, China, Nepal, and Pakistan.9 Fruits of G. trichophylla are known to be used for treatment of various ailments in traditional system of medicine especially treatment for pain and inflammation; leaf oil is used to cure swelling and fracture, however, dried branches are used in making incense fire during religious ceremonies by inhabitants across the Trans Himalaya region of Uttarakhand.10,11 In general, use of wild edibles is now limited to certain remotely located areas and very less is known about their antioxidant and nutritional properties. In this context, present study attempts to analyze fruit samples of G. trichophylla under following objectives:
To estimate polyphenolics and antioxidant activity by different in vitro assays.
To determine nutritional profile of fruits across different altitude sites.
To study the relationship of polyphenolics with antioxidant activity and nutrients.
The outcome of the study is likely to help in prioritization of other wild edible fruits in the Himalayan region and their nutritional potential can be harnessed in local, national and international markets, in such a way to contribute for enhancing the income of hill people in remote hilly areas.
Sample collection
The fresh ripened fruits of Gaultheria trichophylla (Figure 1) were collected from different locations, Martoli bugyal (3475 m ASL, 30º20.539”N/80º11.428”E) and Milam bugyal (3546 m ASL, 30º26.326”N/80º08.836”E) of Trans Himalaya region of Johar valley, Pithoragarh district, Uttarakhand, India. Collated fruits were brought to the laboratory in paper bags and stored at 4ºC until experimentation.


Figure 1 A. Gaultheria trichophylla growing in Trans Himalayan region of Uttarakhand B. colletion of G. tricophylla fruits by inhabitants.
Chemicals and reagents
2, 2-Diphenyl-1-picryhydrazyl (DPPH) radical, Gallic acid, ascorbic acid, quercetin and catechin were purchased from Sigma–Aldrich (Steinheim, Germany). Sodium carbonate, 2-(N-morpholino) ferric chloride, potassium persulphate, potassium acetate, sodium acetate, aluminum chloride, acetic acid and hydrochloric acid from Qualigens (Mumbai, India), and 2,4,6-tri-2-pyridyl-1,3,5-triazin (TPTZ), 2,2-Azinobis-3- ethylbenzthiazoline-6-sulphonic acid (ABTS), methanol and ethanol from Merck Co., (Darmstadt, Germany). All the chemicals purchased were of analytical grade.
Extract preparation
Fresh fruits of Gaultheria trichophylla were cut into four parts than grounded using mortar and pestle to a fine texture. Precisely 20g of each sample was weighed into a conical flask and extracted with 200ml 80% methanolic solution. Briefly, the mixture was homogenized for 1 min using an Ultra-sonicater (Toshiba- India) and kept in water-bath 45ºC for 1h. After that, mixture was centrifuged at room temperature for 20 min at 10,000 rpm and supernatant was removed and filtered by Whatman filter paper no 2 and stored in amber screw-capped glass vials at -20ºC till analysis. All the experiments were performed in Biodiversity Conservation and Management Laboratory of G.B. Pant National Institute of Himalayan Environment and Sustainable Development (GBPNIHESD) Almora, Uttarakhand India.
Estimation of polyphenolics
Total phenolic content: Folin-Ciocalteu’s colorimetric method was used for the estimation of total phenolic content in the methanolic extract of fruits with slight modification.12 In brief, 0.50 ml methanolic extract were diluted with 4.50 ml distilled water and added 0.50 ml Folin-Ciocalteu’s reagent and allowed to reaction for 5 minutes. This mixture was neutralized by 2.50 ml of 7% sodium carbonate (w/v) and kept in dark at room temperature for 90 minutes. The absorbance of resulting blue colour was measured at 765 nm using UV-VIS spectrophotometer (Hitachi U-2001) and results were expressed in mg Gallic acid equivalent (GAE) per gram of fresh weight (FW).
Total tannin content: Total tannin content was estimated following Nwinuka et al.13 with slight modification. Briefly, reaction mixture was prepared with adding (0.25ml) methanolic extract, (2.25ml) distilled water and added (0.50ml) Folin’s Dennis reagent and allowed to reacts for 1 minute. This reaction mixture was neutralized by adding (1.0ml) 7% sodium carbonate (w/v) and kept in water bath in 25ºC for 20 minutes. The absorbance of reaction mixture was measured at 700 nm using UV-VIS spectrophotometer (Hitachi U-2001) and results were expressed in mg tannic acid equivalent (TAE) per gram of fresh weight (FW).
Total flavonoid content: Total flavonoid content in fruit extract was determined by using aluminum chloride colorimetric method with minor modification.14 Briefly, the reaction mixture of (0.50ml) methanolic extract, (1.50ml) distilled water and (0.50ml) 10% (w/v) aluminum chloride, and (0.10ml) 1 M potassium acetate was prepared. This mixture was incubated at room temperature for 30 min and absorbance was measured at 415 nm UV-VIS spectrophotometer. Quantification of total flavonoids was done and results were expressed in mg quercetin equivalent per gram of fresh weight (mg QE/g FW).
Total flavonol content: Total flavonol content in fruit extract was determined using the method of Kumaran et al.15 with slight changes. Briefly, (2.0ml) metahnolic extract, (2.0ml) 2% (w/v) aluminum chloride-ethanolic solution and (3.0ml) 50 g/l sodium acetate solution were mixed and kept it room temperature for 2.5 h. The absorbance of reaction mixture was measured at 440 nm UV-VIS spectrophotometer and results were expressed in mg catechin equivalent per gram of fresh weight (mg CE/g FW).
Estimation of nutrients
Total carbohydrate content: Total carbohydrate content was estimated by followed Hedge et al.16 with minor modification. The fresh fruit sample was hydrolyzing with 2.5N HCL and kept in boiling water bath for three hours and it was neutralized with sodium carbonate. The extract was then centrifuged and the supernatant was collected for analysis and absorbance was taken at 590 nm and result was expressed mg/g FW.
Sodium and potassium content: For quantitative purpose, working standard solution of the elements namely sodium (Na), potassium (K) were prepared from the stock standard solution. Calibration and measurement of Na and K were analyzed on a flame photometer model-Systronics Medi flame 12717 and results were expressed mg/g FW.
Estimation of antioxidant activity
The antioxidant activity was determined using different in vitro methods such as ABTS [2, 2’-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salts], DPPH (1, 1-diphenyl-2-picrylhydrazyl) and FRAP (ferric reducing antioxidant power).2
All the analyzed parameters polyphenolics (i.e., total phenolics, tannins, flavonoids, flavonols), antioxidant activity (i.e., ABTS, DPPH, FRAP assay) and nutritional parameters (i.e., carbohydrate, sodium and potassium) were measured in six replicates. The value for each sample was calculated as mean of all replicates with ± standard error and subjected to paired t test for the separation of means using SPSS software version 16 (SPSS Inc., Chicago IL, USA). Correlation coefficient (r) was also determined using the same software.
Polyphenolic content and antioxidant activity
The analysis of phytochemical reveals that polyphenolic content and antioxidant activity varied significantly (p<0.05) across the sampling locations. The higher content of total phenolics (3.71 mg GAE/g FW), tannins (2.62 mg TAE/g FW), flavonoids (1.75 mg QE/g FW) and flavonols (1.03 mg CE/g FW) was recorded in fruits of Milam bugyal (Figure 2). Similarly, fruits of Milam bugyal were found the best sources of free radical scavenging antioxidant activity (DPPH activity: 2.56 mM AAE/100g FW) and ferric reducing antioxidant activity (FRAP activity: 3.99 mM AAE/100g FW). However, total antioxidant activity (ABTS activity: 4.35 mM AAE/100g FW) was exhibited higher in fruits of Martoli bugyal (Figure 2). Earlier studies have revealed that polyphenolics such as phenolics, flavonoids, tannins are ubiquitous, which showed antioxidant properties, and act as reducing agents, hydrogen donors, free radical scavenger, interrupting chain oxidation reaction which prevent oxidative cell damage.18,19 Present investigation indicates that fruits of higher altitude site are better source of polyphenolics and antioxidant contents. Such types of results have also been reported in fruits of Myrica esculenta,7 Berberis aristata.20 Earlier studies have also suggested that altitude gradient, geographic conditions, biotic and abiotic factors along with several environmental conditions such as temperature, rainfall, maturity at harvest, soil, UV radiation, sunlight, habitat conditions, etc. were also affected the accumulation of secondary metabolites in plants, especially in fruits.21–23 Increased accumulation of bioactive compounds with altitude incline was also reported in several other Himalayan species such as Hedychium spicatum,24 Rosa damascena25 and Valeriana jatamansi.26

Figure 2 Comparison of different parameters across study sites (A) phytochemical: Phenol (mg GAE/g FW), Tannin (mg TAE/g FW), Flavonoid (mg QE/g FW), Flavonol (mg CE/g FW); (B) antioxidant activity : ABTS assay AAE/100g FW), DPPH activity (mM AAE/100g FW), FRAP assay (mM AAE/100g FW) and (C) nutrients: Carbohydrate content (mg/g FW), Sodium (mg/ml FW), Potassium (mg/ml FW). S1 and S2 are study sites, GAE: Gallic Acid Equivalent; TAE: Tannic Acid Equivalent; QE: Quercetin Equivalent; Catechin Equivalent; AAE: Ascorbic Acid Equivalent; FW: Fresh Weight.
Nutritional content
The studied nutritional contents in fruits of Gaultheria trichophylla varied significantly across sites. The fruits of Milam bugyal exhibited significantly higher content of carbohydrate (1.02 mg/g FW), sodium (1.70 mg/g FW) and potassium content (2.17 mg/g FW), as compared to fruits of Martoli bugyal (Figure 2). Nutrients are essential to humans, for structure and development, proper physiological function and regulation process of the body.27 Also, they are important for ionic balance, prevention of chronic diseases28 and disorders like cramps, irregular heart beat and kidney failure.29 Previous studies reported that soil, altitude, precipitation, location specific climatic condition are major factors for affecting chemical constituents in fruits30 and medicinal plants.31
Relationship of attitudinally different sites and analyzed parameters
While establishing relationship between altitude and analyzed parameters (Supplementary Table 1), altitudes showed significant (p<0.01) positive correlation with phenolics (r=0.960), tannins (r=1.00), flavonoids (r=0.975) and flavonols (r=0.953). Likewise, altitude showed strong relationship with DPPH activity (r=0.980) and FRAP activity (r=0.993), however, non-significant (r=-0.931) with ABTS activity. Altitude established positive (p<0.01) relationship with carbohydrate (r=0.931) and sodium (r=0.933) content. Similarly, total phenolic content showed significant (p<0.01) relationship with tannins (r=0.959), flavonoids (r=0.962), flavonols (r=0.952) and antioxidant activity (DPPH and FRAP activity r=0.955 and r=0.931 respectively). As same, phenolics showed significant (p<0.01) linear relationship with antioxidant activity (i.e., DPPH r=0.955; ABTS r=0.894)) and nutritional content (i.e., sodium r=0.903; potassium r=0.862) (Figure 3 and 4). Similar kind of trends also reported in Guava32 and Roscoea procera.33 Elsewhere, studies also indicated that phenolics are positively correlated with antioxidant activity34,35 and responsible for the maximum activity.
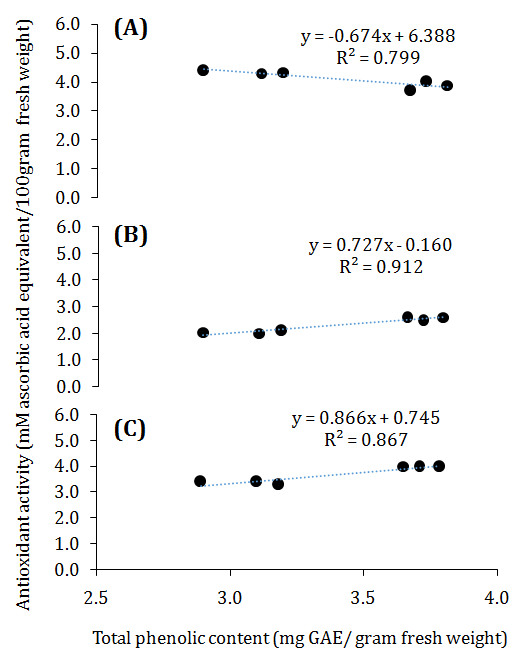
Figure 3 Relationship between total phenolic compounds and antioxidant capacity in G. trichophylla berries of different populations. (A) linear correlation between total phenolic content and antioxidant capacity quantified by ABTS assay, (B) linear correlation between total phenolic content and antioxidant capacity quantified by DPPH assay and linear correlation between total phenolic content and (C) antioxidant capacity quantified by FRAP assay.
Wild edible fruits of Gaultheria trichophylla were found highly nutritious and rich in polyphenolics and antioxidants. Therefore, G. trichophylla can be utilized as a potential source of natural antioxidants and various nutritive products can be developed through sustainable harvesting of fruits in pharmaceutical and nutraceutical industries. Further, fruits can be utilized for preparing juice, jam, sauce and other food supplements. This mechanism will not only contribute in enhancing the economic status of inhabitants but also help in sustaining life in the Trans Himalayan conditions. However, large scale propagation of G. trichophylla in suitable locations needs to be promoted for addressing issues of conservation and sustainable utilization of wild resources.
Authors are grateful to the Director, GB Pant National Institute of Himalayan Environment and Sustainable Development (GBPNIHESD) for facilities. The support received from inhabitants of Milam village is gratefully acknowledged. Funding support from CSIR project (38-1346-12-EMR-II) is gratefully acknowledged. We are grateful to Uttarakhand Forest Department and Indo Tibetan Border Police for logistic and field support.
The authors declare no conflict of interest.

©2017 Bahukhandi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.