eISSN: 2576-4462


Research Article Volume 7 Issue 4
1Rosario Izapa Experimental Station, National Institute of Forestry, Agriculture, and Livestock Research, Tuxtla Chico, Chiapas, México
1Rosario Izapa Experimental Station, National Institute of Forestry, Agriculture, and Livestock Research, Tuxtla Chico, Chiapas, México
2Deparment of Genetics, University of Sao Paulo “Luiz de Queiros”, College of Agriculture. Piracicaba, Sao Paulo, Brazil.
Correspondence: Víctor Hugo Díaz Fuentes. Rosario Izapa Experimental Station. National Institute of Forestry, Agriculture, and Livestock Research. Kilometer 18, Tapachula-Cacahoatán. Tuxtla Chico, Chiapas, Mexico, Tel 8000882222 ext. 86410
Received: December 15, 2023 | Published: December 29, 2023
Citation: Fuentes VHD, Leobardo ID, Hernández BGD. Phenological grown stages of mangostan (Garcinia mangostana L.) in the Soconusco Region, Chiapas Mexico, according to the BBCH extended scale. Horticult Int J. 2023;7(5):178-186. DOI: 10.15406/hij.2023.07.00293
The mangosteen, originating from Southeast Asia, was introduced to Mexico in 1967. Presently, there are over 800 hectares of mangosteen cultivation in Mexico, with approximately 95% located in the Soconusco region, Chiapas. For Mexico and other emerging Latin American countries engaged in mangosteen cultivation, there is a lack of systematic studies on its phenological behavior under the specific local environmental conditions across various fruit-producing regions. The study aimed to identify and characterize mangosteen phenology in the Soconusco region, Chiapas, Mexico. The extended scale of Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie (BBCH) was utilized for this purpose. Additionally, the thermal accumulation in degree-days (GDD) throughout the entire phenological development cycle of mangosteen in the study area was calculated. The study was conducted in a 10-year-old plantation situated in the municipality of Tapachula, Chiapas, from June 21, 2021, to July 20, 2022. The emergence of reproductive buds commenced after a dry period of 34 days. From the emergence of reproductive buds to anthesis, it spanned a period of 34 days, while fruit development after anthesis took 103 days. The thermal accumulation during the complete phenological development cycle of mangosteen amounted to 7,391 GDD. The study's findings enable agronomic management of mangosteen cultivation in the Soconusco region, Chiapas, aligning it with its development in response to local climatic conditions, thereby optimizing yields and profitability.
Keywords: Mangosteen, phenology, Mexico
The mangosteen, native to Southeast Asia, is an evergreen tree belonging to the Clusiaceae Family (syn: Guttiferae). Due to its unique flavor, it has been regarded as one of the most exquisite tropical fruits. Moreover, the discovery of its nutraceutical and medicinal properties has stimulated an increase in its demand in international markets in recent years. Currently, it is cultivated in Thailand, Malaysia, Indonesia, the Philippines, Vietnam, Burma, Cambodia, Singapore, and to a lesser extent in Ivory Coast, Madagascar, Sri Lanka, India, China, and Australia. In the Americas, there are commercial plantations of varying sizes, mainly in Guatemala, Mexico, Colombia, Brazil, Puerto Rico, Honduras, and Panama.1-4 With a cultivated area of 70,900 hectares and a production volume of over 350,000 tons per year, Thailand is the leading global producer and exporter of mangosteen.3,5
Mangosteen was introduced to Mexico in 1967 from Malaysian fruits. However, it was in 2005 that the establishment of commercial mangosteen plantations began, serving as a productive conversion alternative in the country's humid tropical region. Although there are no official statistics, it is estimated that there are currently over 800 hectares cultivated with mangosteen in Mexico, with approximately 95% located in the municipalities of Tuxtla Chico, Tapachula, Huehuetán, Cacahoatán, Metapa, and Frontera Hidalgo in the Soconusco region, Chiapas.4 A significant portion of these plantations has been established in the last 10 years, hence they are just beginning or are about to start their productive stage, typically after 6-7 years of being established in the definitive site.
In its adult stage, the mangosteen tree can reach up to 25 meters in height. The flowers are unisexual, predominantly female, although there are also hermaphroditic flowers, which physiologically behave as female due to their sterile staminoids, rendering the pollen non-viable.6,7 Trees bearing male flowers are unknown; therefore, only female trees have been identified and cultivated. The flowers are found at the ends of the branches. Like other fruit trees, the mangosteen requires a dry period for floral induction.8 The reproductive buds sprout after a dry period of 15 to 30 days,3 although in the Soconusco region, Chiapas, it has been observed that this period can extend up to 42 days after the last rain. The duration of the dry period before flowering impacts production. Production increased as the drought period before flowering extended.9 The fruit is a subglobose berry, usually seedless. The average size of the fruits is 52 mm in diameter, ranging from 32 to 64 mm, with an average weight of 93.7 g, ranging from 25 to 180 g or more.4 Inside the fruit are from four to seven variable-sized ovoid segments arranged in a circle. They consist of a bright white, juicy, sweet, slightly acidic, and fragrant pulp or aril, constituting the edible part of the mangosteen. One or two segments are typically larger and contain a seed-like structure. However, these "seeds" are not actual seeds (sexual embryos) but adventitious embryos (asexual embryos) that develop on the inner carpel wall without fertilization, through a process of asexual reproduction called apomixes.8 The mangosteen is considered an obligate apomict6, and due to this condition, all cultivated mangosteen plants retain the same characteristics as the trees from which the fruits were obtained to extract the seeds for propagation. Hence, all cultivated mangosteen trees are considered a single clone derived from female trees.10 There is a noticeable alternation in flowering and fruiting, which varies from tree to tree and from year to year.11,4
The suitable agroecological conditions for cultivating mangosteen are found in the humid tropics, ranging from sea level to 600 meters in altitude. It can also be cultivated up to 900 meters above sea level, although its growth is less optimal at higher altitudes. It requires annual rainfall exceeding 1,270 mm, an average annual temperature between 25 and 30 °C, and a relative humidity above 80% (18), with a dry period of 15 to 30 days to stimulate flowering.3,12 During the initial growth stages, the foliage is susceptible to sunburn from direct exposure to solar radiation, necessitating shade for the first 2 to 3 years. The most suitable soils for its cultivation are porous, deep, with high organic matter content, and a pH ranging from 5 to 6.5.3,8
In the Soconusco region in the State of Chiapas, Mexico, mangosteen plantations are distributed in an altitude range of 20 to 900 meters. This altitudinal variation significantly affects the vegetative and reproductive development stages of the mangosteen in this region. In plantations located at altitudes below 400 meters, the productive stage commences at 6 years.
Flowering occurs in December, fruiting starts from January, harvesting begins from the second half of April, and concludes in July-August. In plantations situated at altitudes between 400 to 900 meters, the productive stage commences after 7 years. Flowering takes place in the February-March period, fruiting in April-May, harvesting starts in June-July, and concludes in September, occasionally extending into October. This variation in the occurrence periods of different developmental stages of mangosteen in the Soconusco region, impacts agronomic management, fruit quality, market prices, and thereby influences the crop's profitability.
In cultivated species, knowledge about the phenological behavior of the crop is essential for the timely programming and implementation of different agronomic management practices such as irrigation, pruning, fertilization, pest control and harvest.13 The periodic biological events corresponding to the specific stages of development of plant species are determined mainly by temperature, which regulates the rate of vegetative growth and reproductive development,14 because the progression of the different stages of its development is due to the accumulation of fundamentally thermal stimuli.15 Each species has a specific temperature requirement before certain phenological stages are reached.16 One of the most widely used methods to determine the thermal requirements of each species is the accumulation of average daily temperature above a base temperature (Tb). The temperature required for an organism to reach full development is called physiological time and is calculated in accumulated growing degree-days (GDD). A degree day is equivalent to one degree above a base temperature.17 For the onset and completion of each phenological phase, plants require a minimum accumulation of temperature (expressed in GDD). This requirement varies among species. The duration of a physiological phase is expressed as the sum of the GDD allow determining the effects of temperature and timing of biological processes.18
In 1974, Zadoks19 proposed the first decimal code to standardize the description of corresponding development stages across different crops. Building upon this, Bleiholder20 in 1989 developed the Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie (BBCH) scale. The BBCH scale is a system for uniformly coding the phenological identification of growth stages for all species of mono- and dicotyledonous plants. To achieve this, the scale uses clearly recognizable and common external characteristics among different plant species. The BBCH scale employs a decimal coding system. The complete cycle of plant development is divided into ten principals and ten secondary stages, all clearly distinguishable. The principal developmental stages are identified using numbers from 0 to 9 in ascending order and include a description of the state. The secondary stages precisely describe short phases of development that occur within a specific principal stage. They are also coded using numbers from 0 to 9 or a percentage value. The combination of the numbers from a principal growth stage and the number from a secondary stage forms the 2-digit code utilized by the scale. The BBCH scale was further expanded in 1992 by Hack21 and termed the extended BBCH scale. The extended BBCH scale, represented by three digits, provides more details on plant development as it includes the numbered mesostadia from 0 to n, which are incorporated between the primary and secondary stages. Due to its ease of application, the extended BBCH scale is currently the most widely used uniform identification and coding system for the different growth stages of both mono- and dicotyledonous plant species. In tropical fruit trees, it has been utilized in phenological studies on citrus,22 sugar apple,23 pineapple,24 sapota,25 jackfruit,26 mango,27 and mangosteen28 among others.
The phenology of mangosteen or different aspects related to it has been described by several autors.28-35 All these studies have been conducted mainly in Asian countries, which are traditionally mangosteen producers. However, for emerging countries in mangosteen cultivation in Latin America, such as Mexico, there are no studies on the phenological behavior of mangosteen under local conditions. Accordingly, the objective of the present study was to identify and describe, using the extended BBCH scale, the phenology of mangosteen in the Soconusco region, Chiapas, Mexico. This knowledge is not only crucial for guiding the agronomic management of mangosteen based on its phenological behavior, but also for anticipating potential variations in this behavior within the future context of global climate change.
The study was conducted in a 10-year-old plantation located in Viva México, Tapachula, Chiapas, from June 21, 2021, to July 20, 2022, encompassing a mangosteen production cycle from the conclusion of the 2021 harvest to the end of the 2022 harvest. The plantation is situated at an altitude of 70 m. The prevailing climate in the area is warm and humid, with summer rains, an average annual temperature of 27.5°C, an annual average minimum of 21°C, and an average maximum of 34.1°C. The annual average precipitation is 2,065 mm. The plantation has a tree spacing of 8 x 8 m between rows and plants (156 trees ha-1). It began its productive phase at 6 years of age and features a sprinkler irrigation system mainly applied during the dry period (January-April).
Ten random trees were selected, and four branches per tree were chosen, totaling 48 vegetative buds and 42 reproductive buds. Descriptive data regarding the vegetative and reproductive development of the selected trees were recorded according to the three-digit extended BBCH scale, which includes mesostages between primary and secondary growth stages. Seven out of the ten main stages of BBCH development were coded and described: vegetative bud development (Stage 0), leaf development (Stage 1), shoot development (Stage 3), reproductive development (Stage 5), flowering (Stage 6), fruit development (Stage 7), and fruit maturity (Stage 8). Data for stages 2 (formation of side shoots or tillering) and development of harvestable vegetative (Stage 4) indicated in the extended BBCH scale were not recorded, as both stages do not occur in mangosteen. Primary growth stages were subdivided into secondary growth stages represented by codes from 0 to 9, identifying and describing a specific primary growth stage with ordinal or percentage values. Meso-stages were also described and coded from 0 to n, detailing different vegetative and reproductive flows observed between a primary and secondary stage. Each growth stage was recorded with its corresponding code, description, and photographic image. Data for the vegetative bud development (Stage 0), leaf development (Stage 1), shoot development (Stage 3), flowering (Stage 6), and fruit maturity (Stage 7) were recorded every third day. Data concerning fruit development (Stage 5) were recorded every 7 days.
The daily records of precipitation, average temperature, maximum, and minimum temperature during the study period were obtained from the Tapachula Meteorological Observatory, belonging to the National Water Commission (CONAGUA), located 5 kilometers from the study plantation. GDD for each phenophase was calculated using the daily maximum and minimum temperatures through the following formula proposal by Ometto,36 with a base temperature of 12.8°C and an upper threshold temperature of 36°C, used by Awachare et al.28
GDD = (TM + Tm)/2 – Tb, where:
GDD = Accumulated Growing Degree Days
TM = Maximum Temperature
Tm = Minimum Temperature
Tb = Base Temperature
According to the extended BBCH scale, seven primary growth stages and 38 secondary stages with their corresponding mesostages were identified, which are described below and illustrated in Figures 1-3.
Principal growth Stage 0: Vegetative bud development
The vegetative bud development of mangosteen (stage 0) occurs in two - three flushes during the period from July to October. Ceases in the first week of November when the tree enters a state of dormancy due to decreased precipitation. Starts with the budding and swelling of the vegetative bud and concludes when the first leaves, which remain unseparated, have fully emerged. The complete process of this stage spans over 4 weeks, progressing through the phases described below Figure 1

Figure 1 Principal growth stage of mangosteen; vegetative bud development (stage 0); leaf development (stage 1) and shoot development (stage 3), according to the extended BBCH scale.
Principal growth Stage 1: Leaf development
During the annual development cycle of the mangosteen, leaf development occurs in two phases. The first begins in August, interrupted by decreased precipitation in November, inducing dormancy in the tree. Another leaf development phase occurs from the second half of January to the second half of February. The entire leaf development process spans approximately 5-6 weeks and includes the stages described below, illustrated in Figure 2.

Figure 2 Principal growth stage of mangosteen; reproductive development (stage 5) and flowering (stage 6) according to the extended BBCH scale.
Principal growth Stage 3: Shoot development
The development of the second leaf cycle in mangosteen is sequential to the first cycle. It occurs in successive flushes during the periods of September, January-february and may-june. It starts with the emergence of the new shoot between the leaves of the first leaf cycle and concludes when the new shoot has reached its maximum length, and its leaves have turned dark green. The complete leaf development spans a period of 5-6 weeks and includes the stages described below, illustrated in Figure 2.
Principal growth stage 5: Reproductive development
The reproductive stage begins in the first week of december, following a dry period of 34 days, and concludes in the month of february. This phase signifies the end of the tree's dormant period. From the emergence of reproductive buds to anthesis, mangosteen flowers take 30 to 35 days.37 In our study, this period lasted 34 days. It commences with the emergence of the reproductive bud at the branch's growth point and concludes when the petals of the new flower within the floral bud are observed, yet to fully unfold. The entire process of reproductive development spans a period of 4 weeks across the stages described below and illustrated in Figure 3.

Figure 3 Principal growth stage of mangosteen: fruit development (stage 7) and fruit maturity (stage 8) according to the extended BBCH scale.
Principal growth stage 6: Flowering
The flowers of the mangosteen arise at the ends of the branches (terminals), either solitary or in groups of three, although some trees produce clusters of up to 12 flowers.7 They are functionally female since, despite having staminoids, the pollen isn't fertile.38 They last a short time, opening in the afternoon and shedding petals shortly after.38,39 The complete process of flowering (stage 6) spans a period of 4-5 days across the stages described below and illustrated in Figure 3.
Principal growth stage 7: Fruit development
Depending on climatic conditions and cultivation practices, fruit development spans a period of 115-180 days after anthesis.40 In our study, this phase lasted 103 days. During the first week after anthesis, fruit growth is slow. In its initial stages, fruit growth mainly corresponds to pericarp development. Dry matter accumulation in the aril increases 20 days after anthesis and continues throughout fruit development. From the 2nd week after anthesis until week 10, growth is rapid. During this period, the fruit reaches its peak content of pulp, rind, sugars, and acidity.41 Subsequently, the growth rate decreases, and after week 13, growth stop.40 Fruit development encompasses the stages described below and illustrated in Figure 4.
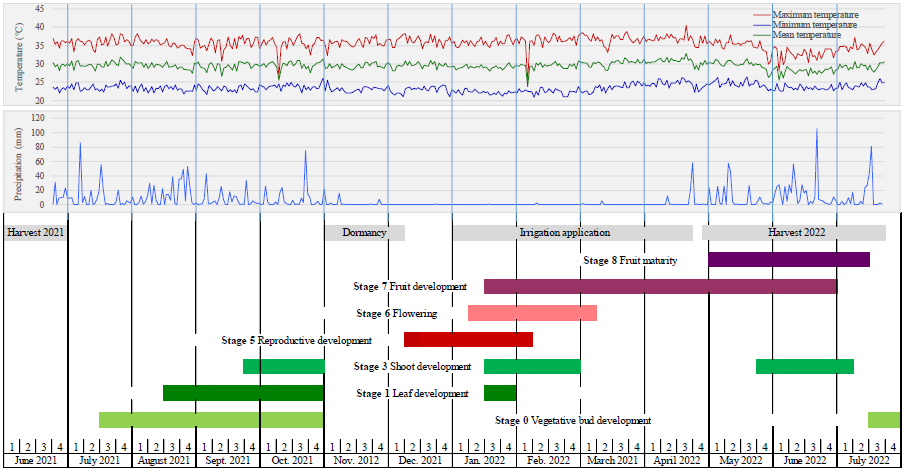
Figure 4 Prevalent precipitation (mm) and maximum, minimum, and average temperatures (°C) during the phenological growth stages of mangosteen (Garcinia mangostana L.) in the Soconusco Region, Chiapas, Mexico, according to the extended BBCH scale.
Principal growth stage 8: Fruit maturity
The pericarp color serves as the primary criterion for determining mangosteen fruit maturity. Typically, fruits are harvested from the stage they exhibit a light greenish-yellow color with scattered pink spots until the pericarp turns dark purple. The maturation phase begins when the pericarp shows a greenish-yellow hue with 5% scattered pink spots43. Harvesting at this maturation stage does not negatively impact the final fruit quality, offering a slightly longer shelf life compared to fruits harvested at later stages. From this initial stage, the maturation process is already stimulated. After harvesting the fruit at this maturation state, it triggers and continues for subsequent stages. The maturation phase until the fruit reaches full maturity (with a dark purple pericarp) spans a 9-day period, consistent with our study findings. To identify the different fruit maturation stages, we used, with slight modification, the pericarp color index proposed by Palapol et al.,42 and includes 6 stages described below and illustrated in Figure 5.
The vegetative development of mangosteen (stage 0) commences in July and concludes in the last week of October when the tree enters a dormant state due to a drastic reduction in precipitation. Drought-induced stress results in the accumulation of carbohydrates in the leaves, reserved for subsequent use during flowering and fruiting.43,44 This vegetative development is crucial for productivity as many branches developed during this phase become productive, bearing fruit in the subsequent production cycle. In the second half of January, vegetative growth (stages 1 and 3) recommences and continues until the fourth week of February. The development of the second leaf cycle (stage 3) and subsequent ones occurs again during the May-June period. Except for this brief period of secondary vegetative growth, during fruit development (stage 7) there is no vegetative growth. Similar to other plant species, vegetative growth in mangosteen is primarily influenced by rainfall rather than temperature. As shown in Figure 1, stages 0 (vegetative bud development), 1 (leaf development), and 3 (shoot development) occur under high precipitation conditions (1661 mm recorded during the encompassed period), with minimal variations in average temperatures (25 to 31°C).
The initial reproductive buds (stage 5) emerge during the second and third weeks of December, following a dry period of 35 days after the last rainfall. Prevailing average temperatures of 29°C, maximums of 36°C, and minimums of 22.6°C were recorded during the 30 days preceding the budding of the reproductive buds (Figure 1). This aligns with others observations,32 indicating that the minimum temperature triggering mangosteen flowering is 21°C. Mangosteen trees are resistant to water scarcity but not to drought; hence, it's essential to avoid water deficit stress, necessitating watering during the dry period.43 At the study plantation irrigation was applied from the first week of January throughout the dry period until the fourth week of April to prevent flower abortion during stage 5 and small fruit during stage 6 and promote fruit development (stage 7). During the flowering, fruit development, and ripening phases, the maximum temperatures recorded were 36°C, 36.6°C, and 34.2°C, respectively; minimums were 22.4°C, 23.2°C, and 24°C, respectively, and averages were 29.2, 29.9, and 29, respectively. These values are relatively similar to those reported by other authors28,9 in studies conducted on mangosteen phenology in the state of Karnataka, India, and the impact of climate on mangosteen production in Thailand. The thermal accumulation in degree-days (GDD) recorded during the complete phenological development cycle of mangosteen in the study area was 7,391 GDD (Table 1). This value is higher than that obtained (7114 GDD) in the phenological study on mangosteen in the state of Karnataka, India.28 Differences in phenophases concerning thermal accumulation among different regions could be attributed to altitude and latitude, which are the primary factors influencing temperature regimes, while other factors hold less significance compared to the temperature effect.45 The thermal accumulation during fruit development and maturation phases are relatively similar to those reported in studies conducted in plantations in India28 and southern Thailand.31
|
Principal growth stage |
BBCH Code |
Substages |
Duration (days) |
Accumulative growing degree days (GDD) |
|
Vegetative bud development |
0 |
010 - 019 |
107 |
1,595 |
|
Leaf development |
1 |
110 - 119 |
92 |
1,163 |
|
Shoot development |
3 |
311 - 319 |
134 |
1,935 |
|
Reproductive development |
5 |
510 - 519 |
67 |
935 |
|
Flowering |
6 |
610 - 619 |
59 |
773 |
|
Fuit development |
7 |
710 - 719 |
96 |
943 |
|
Fruit maturity |
8 |
810 -819 |
81 |
1210 |
|
Total Degree Days |
7,391 |
Table 1 Duration (days) and thermal accumulation (GDD) of the phenological phases of mangosteen in the Soconusco region, Chiapas, Mexico.
To the National Institute of Forestry, Agriculture, and Livestock Research of Mexico, for funding this study.
To Engineer Abel Méndez Ávila and Adán Colomo Remires for the facilities and support provided to carry out the fieldwork for the study.
The authors declare no conflict of interest exists.

©2023 Fuentes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.