eISSN: 2576-4462


Research Article Volume 4 Issue 5
Graduate Program in Genetics and Molecular Biology, Eestadual University of Santacruz, Brazil
Correspondence: Jose Julian Apraez Muñoz, Graduate Program in Genetics and Molecular Biology, Eestadual University of Santacruz, Brazil
Received: August 10, 2020 | Published: September 2, 2020
Citation: Muñoz JJA, Almeida AAF, Ahnert D, et al. Mitigation of Pb toxicity by Zn in young plants of the cacao clonal CCN 51 genotype grown in soil: physiological, biochemical, nutritional and molecular responses. Horticult Int J. 2020;4(5):156-167. DOI: 10.15406/hij.2020.04.00176
The soil is among the main contamination sources of Pb in cocoa beans, which carries potential risks to human health from ingesting contaminated cocoa products. Therefore, the Pb contents in cocoa beans depend not only on the genotype, but also on the geographic location. Pb toxicity in plants is highly modified by increasing the Zn/Pb ratio. Pb uptake by the roots decreases with the increase in the Zn content in hyperaccumulator plant species of Pb/Zn, as well as in non-accumulator species, clearly indicating that the inflow of Pbis largely attributed to Zn transporters, with a strong preference for Zn at the Pb detriment. The objective of this study was to evaluate the influence of Zn on mitigation of Pb toxicity in young plants of the cacao clonal CCN 51 genotype grown in soils with different doses of Pb, Zn and Zn+Pb, through physiological, biochemical, molecular and nutritional responses. Young plants of the clonal cacao genotype CCN 51 grown in soils with high Pb, Zn and Zn + Pb contents accumulate these heavy metals in the roots and leaves. The uptake of Pb and Zn by the roots and their transport to the aerial part promoted significant physiological alterations in variables such as photosynthesis, gas exchange, carboxylation efficiency, nutritional balance, antioxidant metabolism and the genetic expression especially of PsbA and PsbOgenes related to photosystem two efficiency (PSII). The increased activity of the SOD enzyme and the proline content in the leaves contributed to mitigate the toxicities of Pb and Zn at the highest doses of these metallic elements applied in the soil. Furthermore, the adequate doses of Zn + Pb applied in the soil mitigated the toxicity of Pb in the plants. On the other hand, the doses of Zn + Pb and Zn applied to the soil, induced the death of young plants of the clonal cocoa CCN 51 genotype 15 days after the application of the treatments, the application of Zn in adequate doses to the soil. It can be used to mitigate the toxicity of Pb in young plants of the CCN 51 clonal cocoa genotype that grows in contaminated soils.
Keywords: metal toxicity, photosynthesis, antioxidant metabolism, gene expression, mineral nutrients
Theobroma cacao L. is a woody species that is typical of tropical climate and preferably allogamous. It previously belonged to the family Sterculiaceae,1 but was reclassified into the Malvaceae family.2 Under natural conditions, the cocoa tree can reach 20 to 25 m in height,3 while under cultivation it normally varies from 3 to 5 m in height. The geografical origin of cocoa is South America, where several wild populations can be found in the regions of Amazonia and Guianas.4 It is considered the most important perennial crop on the planet, with an estimated global production of more than 4 million tons,5 and is cultivated predominantly in tropical areas of South America, Central America, Asia and Africa.6
Lead (Pb) is found naturally in the earth's crust. However, the levels of Pb in the soil have been increasing through anthropogenic actions in industries such as battery manufacturing, metal mining and smelting,7 urban and industrial waste, fertilizers, pesticides, and additives.8 Pb has no known biological function, so it is not an essential element for plants. However, many studies have shown that certain plant species can absorb it and then show signs of toxicity.9 The absorption of Pb by the roots is regulated by pH, particle size and cation exchange capacity in the soil, as well as by exudation and other physicochemical parameters.10 Once absorbed by the plants, Pb causes several direct and indirect effects on seed germination, growth and metabolism.11 As its concentration in the soil increases, Pb can begin to impair the ultrastructure of subcellular components such as chloroplast, mitochondria, nucleus and membranes in plants. This damage can induce loss of function in the organelles and eventually affect normal physiological functions such as photosynthesis, respiration, protein synthesis, cell division, inducing programmed cell death.12
The plants either diminish or neutralize the effects of Pb by means of specific mechanisms of protein and non-protein origin. The cell wall is the first barrier to prevent damage to the cells by containing pectin with certain carboxylic groups, polysaccharides and proteins that fix Pb ions, reducing movement between the cytoplasm and avoiding damage of the protoplast.9 Besides that, there are non-protein thiols such as phytochelatin, which is a family of cysteine-rich peptides that are enzymatically synthesized from glutathione (GSH). Phytochelatins play an important role in preventing oxidative stress in plant cells, in addition to being involved in detoxification and accumulation of several metals in vacuoles, including Pb.10 In addition to phytoquelatins, there is the role of metallothioneins and of antioxidant metabolism involved in the elimination of reactive oxygen species (ROS). Fuctions of metallothioneins (MTs) resemble phytochelatins; they have high affinity for many metals, including Pb. However, they are low-molecular-weight proteins with high cysteine content, and are overexpressed when organisms are subjected to high concentrations of metal.13 Enzymes of antioxidative metabolism, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidases (GPX) among others, can attenuate the ROS effects.14
Pb-induced toxicity may either inhibit or activate the activity of enzymes involved in antioxidative metabolism and influence the expression and synthesis of these enzymes. However, these actions will depend on metal speciation, plant species and duration and/or intensity of treatment.15 The activity of these enzymes maintains the integrity of cell membranes and important molecules, such as DNA and proteins, avoiding lipid peroxidation and cell death, and therefore reducing the damage caused by Pb.14 Scientific research has recently demonstrated the presence of Pb in soils used for cocoa grown and in products derived of cocoa beans, the example of chocolate.16 The presence of Pb in the soil can be justified by the original rock during soil genesis, by the use of phosphate fertilizers and Cu-based fungicides. These fungicides are applied by cocoa producers with the aim to reduce the attack of diseases such as brown rot and witch's broom, which have Phythophotora sp and Moniliophtoraperniciosa as causal agents, respectively.The present study had with objective main to evaluate the influence of Zn on mitigation of Pb toxicity in young plants of the CCN 51 clonal cocoa genotype, cultivated in the soil with different concentrations of Pb, Zn and Zn+Pb, through of physiological, biochemical, molecular and nutritional responses.
Plant material and growing conditions
The experiment was carried out in the greenhouse at the State University of Santa Cruz (UESC), Ilhéus, Bahia, Brazil (14 ° 47 'S, 39 ° 10' W). SENA tecnoparque Pitalito CCN51 clonal genotype of cocoa used present desirable agronomic characteristics such as high productivity, self-compatibility, witches' broom disease tolerance, and high butter content in its beans, besides being a widely used genotype by cocoa producers in Latin America.17 Clone CCN 51 was selected in Ecuador as the result of crossing the IMC 67 x ICS 95 hybrid with an Ecuadorian cultivar known as 'Canellos'. It is a cultivar that has been widely used in Ecuador and is being used as a parent in many cocoa breeding and selection programs in other countries.17
The clonal plants were obtained from the rooting of stem cuttings from the ends of plagiotropic branches at the beginning of secondary growth, containing the apical bud, three auxiliary buds and three leaves, taken from five to ten years old parent plants. The base of stem cuttings (~3 cm) was dipped into chemically inert talcum powder containing indol-3-butyric acid (IBA) at 4 g kg-1. Afterwards, each stem cutting was transferred to a 288-cm3tubelike, black plastic pot containing organic substrate (grinded Pinus sp. barks and grinded coconut fiber at 1:1 ratio), enriched with macro and micronutrients, according to the recommendations for cacao young plants.
After rooting (4 to 5 months of age), the young plants of clonal cocoa were transplanted for drilled plastic vessels with a capacity of 20 L. Based of utilized soil characteristic (Table 1), proceeding the its fertilization with N, P and K (Supplementary material) and a mixture of CaCO3 and MgCO3, important to reach the Ca+2: Mg+2 4:1 ratio, and raising the soil base saturation value to 30%, which results in increased pH (4.7) for optimum growth conditions for cocoa. The level of fertilization for cacao was based on the needs of the crop during the 120 days of the experiment (Souza Junior, 2008). When the plants were four months old, the treatments of Pb, Zn and Zn+Pb in the soil were applied in a volume of 500 mL/pot, with the following concentrations: treatment 1 (T1, 0.3 g Zn + 0.5 g Pb kg-1 soil), treatment 2 (T2, 0.15 g Zn + 0.75 g Pb kg-1 soil), treatment 3 (T3, 0.45 g Zn + 0.25 g Pb kg-1 soil), treatment 4 (T4, 1.0 g Pb kg-1 soil) and treatment 5 (T5, 0.6 g Zn kg-1soil), together with the treatment 0 (T0, control, without addition of Zn and Pb in soil), totaling six treatments and having ZnCl2 and PbCl2 as sources of Zn e Pb, respectively.
|
pH |
H2O |
4.7 |
|
P |
mg dm-3 |
5.9 |
|
K |
mg dm-3 |
22 |
|
Na |
mg dm-3 |
9 |
|
Ca+2 |
cmolc dm-3 |
1.1 |
|
Mg+2 |
cmolc dm-3 |
0.6 |
|
Al+3 |
cmolc dm-3 |
0.5 |
|
H+Al |
cmolc dm-3 |
4.13 |
|
CEC (t) |
cmolc dm-3 |
2.3 |
|
CEC (T) |
cmolc dm-3 |
5.9 |
|
V |
% |
30 |
|
M |
% |
22 |
|
ISNa |
% |
1.7 |
|
OM |
dag kg-1 |
2.29 |
|
P-rem |
mg L-1 |
38.4 |
|
Zn |
mg dm-3 |
2.3 |
|
Fe |
mg dm-3 |
145.1 |
|
Mn |
mg dm-3 |
15.3 |
|
Cu |
mg dm-3 |
1.5 |
|
B |
mg dm-3 |
0.2 |
|
S |
mg dm-3 |
15.9 |
Table 1 Physical and chemical characteristics of the substrate for growth of cacao plants. P, Na, K, Fe, Zn, Mn, Cu (extracted by Mehlich 1), Ca, Mg, Al (extracted by KCl, 1 M) H + Al (extracted by Ca-acetate 0.5 M, pH 7.0), B (extracted by hot water). SB, sum of bases; t, effective cation exchange capacity; T, cation exchange capacity (pH 7.0); V, base saturation; m, Al saturation; NaSI, Na saturation index; OM, organic matter= Org C.×1.724; P-rem, remaining phosphorus;TS, total sand
During the whole experimental period, the young plants of clonal cocoa were watered with rainwater previously stored. Besides that, photosynthetically active radiation (PAR), temperature and relative humidity inside the greenhouse was continuously monitored and recorded by means of micrometeorological sensors (Hobo H8 Pro Series, Onset, USA). PAR, temperature and relative humidity means recorded during this period were 6±0.5 mol m-2 day-1, 27±0.4°C and 78±0.7%, respectively.
Leaf gas exchange
During the experimental period, the net photosynthetic rate per unit leaf area (A), stomatal conductance to water vapor (gs) and leaf transpiration (E) were measured at 0, 15 and 30 days after application of treatments (AAT), between 8 and 12 h, in a fully expanded and mature leaf. Five plants per treatment were evaluates using a portable LI-6400 photosynthesis measurement system (Li-Cor, Nebraska, USA), equipped with a 6400-02B RedBlue artificial light source. The photosynthetic photon flux density and leaf temperature was set at 800 μmol photons m-2 s-1 and 26°C, respectively, using equipment accessories. The readings were record in the range of 2-3 min (coefficient of variation from 0.1% to 0.2%). The values of A, gs and E were used to calculate the intrinsic (A/gs) and instantaneous (A/E) efficiencies of water use and instantaneous carboxylation efficiency (A/Ci).
Chlorophyll fluorescence
Measurements of chlorophyll fluorescence emission was make on the same leaves used for gas exchange measurements, using a portable fluorometer (Pocket PEA Chlorophyll Fluorimeter - v 1.10 - Hansatech Instruments, Norfolk, UK), between 8 and 12 h. The selected leaves were adapted to the dark for a period of at least 20 min, for reflection of incident solar radiation, decrease of leaf temperature and oxidation of the entire photosynthetic electron transport system, using appropriate clips. After dark adaptation, the leaf tissue was illuminated with a weak-modulated measuring beam (0.25 kHz, < 0.1 μmol m-2 s-1, 650 nm, 1 s) to obtain the minimal fluorescence (F0). A saturating white-light pulse (20 kHz; 3500 μmol m-2 s-1, 650 nm, 1 s) was applied to ensure the variable (FV) and maximum (Fm) fluorescences emissions. The maximum quantum yield of photosystem 2 (Fv/Fm) was calculated as [Fv/Fm = (Fm − F0)/Fm)].19 The fluorescence emission signals were recorded in the acquisition system of Pocket PEA data, using specific software.
Minerals nutrients and Pb
At the end of the experiment, 30 days AAT, samples composed of roots and leaves were collected from different treatments. The dried and ground plant material were weigh out in triplicate (200 mg per sample) and placed in a 50 mL digestion tubes containing 3 mL of concentrated HNO3 (Merck). The tubes were capped with a cold finger, containing distilled water. During the digestion of the samples, the block temperature was gradually increased: (i) 50°C for 30 min, (ii) 80°C for 60 min, and (iii) 130°C for 45 min, plus 1 mL of 30% hydrogen peroxide (Merck). Soon after, the 30% hydrogen peroxide was added for a further 2x (1 mL) at 20 min intervals. Subsequently, after 15 min of the last addition of hydrogen peroxide, the digester block was turn off. When samples reached room temperature, they were transfer into Falcon tubes and volumated to 15 mL with ultrapure water. Subsequently, the macro and micronutrient content and Pbwere analyzed in an Inductively-Coupled Plasma Optical Emission Spectrometer (ICP-OES) model Varian 710-ES.
Enzyms of antioxidative metabolism
In order to perform the enzymatic analysis at leaf level, the plant tissue was collected at 0, 3, 6, 12, 24, 48 and 96 h AAT, immersed in liquid nitrogen and stored in ultrafreezer - 80°C and subsequently lyophilized and stored in a freezer - 20°C. At the time of analysis, the plant material was macerated in liquid nitrogen together with polyvinylpyrrolidone (PVP), to prevent oxidation of the macerate, and stored in ependorff. Immediately afterwards, the macerate was resuspended in extraction buffer (50 mM sodium phosphate buffer, pH 7.0 or 50 mM potassium phosphate buffer, pH 6.0) which varied according to the type of enzyme involved in the antioxidative metabolism, followed by shaking. Subsequently, the material was subjected to sonication, followed by centrifugation. Finally, the supernatant was collected, considering it as the crude extract, which was transferred to a 2 mL microtube, kept in Styrofoam with ice, and used immediately.
The activity of the enzymes guaiacol peroxidase (GPX, EC 1.11.1.7), ascorbate peroxidase (APX, EC 1.11.1.11), superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), polyphenol oxidase (PPO, EC 1.10.3.1) was determined according to the methodological procedures described by Amako et al. (1994), Nakano and Asada (1981), Siddiq et al.20 and Yao et al.21 respectively. The sample and standard readings were done with a UV–vis spectrophotometer (SpectraMax Paradigm Multi-Mode Microplate Reader, Molecular Device, USA).
Proline
Samples lyophilized of second or third mature leaf from the stem apex (approximately 100 mg), collected 30 days AAT, were macerated in liquid N. Immediately after, the proline was extracted by adding 3% (w/v) sulfosalicylic acid to the samples of plant material. Then, samples were centrifuged and the resulting supernatant was used to determine proline concentration, according to the procedures described by Bates et al.22 with minor modifications.23 Trials were performed in triplicates for each biological replicate.
Gene expression
Mature leaves were collected at intervals of 0, 3, 6, 12, 24, 48 and 96 h AAT. Samples were stored at - 80°C after fixation in liquid nitrogen and then lyophilized. For RNA extraction, RNAqueous® kit (Ambion) was use, following the manufacturer's recommendations. RNA samples were used for the synthesis of the cDNA with RevertAid ™ H Minus M-MuLV Reverse Transcriptase, according to the manufacturer's instructions, using oligod (T) 18 primers. Analyzes of qPCR were performed on an Applied Biosystems 7500 Real-Time PCR System thermocycler using non-specific detection sequence (fluorophore) SYBR Green I. The reaction mix were composed of cDNA as a template, 0.5 μM of each primer and 12.5 μL of Maxima® SYBR Green/ROX qPCR Master Mix (2x). The temperature of PCR products was raised from 55 to 99°C at a rate of 1°C/5s, and the resulting data were analyzed using the Light Cycler software.
We have only observed a single band with a characteristic melting point for each sample, indicating that the qPCR had produced a specific product for each used primer-pair. In order to confirm that the qPCR had only produced the genes of interest, the PCR products were calculated and visualized in agarose gel at 1%. The relative expression numbers of the genes were calculating as the number of times in relation to the control plant using the 2-ΔΔCt method.24 Two endogenous genes were used as control in order to detect changes, actin and β-Tubulin. The abundance of transcripts was analyzed using specific primers (Table 2). In order to test the quality of these primers, the specificity and identity of the reverse transcription products, we have monitored the qPCR products after each PCR, using a melt-curve analysis distinguishing gene-specific from non-specific products.
|
Gene |
Acess |
Function |
Primer |
|
psbA |
NC_014676.2C |
Biosynthesis of the psbA protein or D1 protein. |
F-5’GGTTTGCACTTTTACCCGA-3’ R-5´CTCATAAGGACCGCCATT-3´
|
|
psbO |
CL 326conting1a |
Biosynthesis of the psbO protein. |
F-5’GCAAACGCTGAAGGAGTT-3´ R-5’GGCTTGAAGGCAAATGAGTC-3´ |
|
Tpr |
Q5NBT9 |
Associates both with chromatin in the HSP70 promoter and with mRNAs transcribed from this promoter under stress-induced conditions |
F-5’ATGAGCAGCTAAAGCAGGGAA-3´ R-5’TTCCCTCCTTTACCTGCTCAT-3 |
|
Met |
|
Ability to bind to both heavy physiological metals and xenobiotics. |
|
|
Βtubulina |
GU570572.1C |
Endogen |
F-5´TGCAACCATGAGTGGTGTCA-3´ R-5´CAGACGAGGGAAAGGAATGA-3´ |
|
Actina |
Xm_ 018128615c |
Endogen |
F-5´TCCTCTTCCAGCCATCTCTC-3´ R-5´TCTCCTTGCTCATTCGGTCT-3´ |
Table 2 Pairs gene-specific primers that were used in qRT-PCR analysis
Statistical analysis
The experimental design used was the randomized blocks with six treatments, ten replicates and one plant per experimental unit, making a total of 60 clonal plants. Five replicates were used for the measurements of photosynthetics parameters, concentrations determinations of minerals nutrients and Pb and biochemical and molecular analysis. The data were submitted to ANOVA and the means values were compared by Tukey test (p<0.01 and p<0.05).
Pb concentration
Pb accumulations in the roots and leaves of the young clonal cacao plants were found to vary according to the doses of Pb, Zn and Zn+Pb applied to the soil. Treatments T1 (0.3 g Zn + 0.5 g Pb kg-1 soil) and T4 (1 g Pb kg-1 soil) showed higher Pb accumulations in the roots (843.05 and 1112.2 mg dm-3 DW, respectively). On the other hand, T0 (control) and T5 (0.6 g Zn kg-1 soil) (i.e., with no application of Pb to the soil) did not show Pb accumulation in the roots and leaves (Tables 3 and 4). Leaf Pb accumulation exhibited the same tendency as in the roots; for instance, T4 and T1 showed very high values of Pb contents in these organs (3269.70 and 2089.6 mg dm-3 DW, respectively). However, the accumulation of Pb in the leaveswas twice higher than in the roots. There were no significant statistical differences in Pb concentration between T0 and T5 since Pbwas not applied in these treatments (Tables 3 & 4).
|
N |
P |
K |
Mg |
S |
Zn |
Mn |
Pb |
Cu |
Fe |
B |
|
|
TRAT |
-----------------------cmolc dm-3------------------ |
-------------------------------mg dm-3------------------------ |
|||||||||
|
0 |
2.96a |
0.08c |
0.94b |
0.0c |
0.13b |
20.12d |
38.18d |
0.0e |
0.53b |
101.32d |
19.72c |
|
1 |
2.9a |
0.08c |
1.09b |
0.0c |
0.15b |
25.87d |
63.68c |
843.05a |
0.58b |
142.38c |
21.77c |
|
2 |
3.1a |
0.15b |
1.96a |
0.25bc |
0.17a |
217.47b |
244.07b |
206.08bc |
1.40ab |
193.27c |
25.18b |
|
3 |
2.84a |
0.33a |
2.4a |
0.79a |
0.23a |
1520.27a |
680.88a |
155.92cd |
4.05a |
921.47b |
43.40a |
|
4 |
2.79ab |
0.25a |
1.81b |
0.66b |
0.18a |
69.9c |
604.55a |
1112.2a |
2.90a |
1105.57b |
26.58b |
|
5 |
2.47ab |
0.21b |
2.03b |
0.54b |
0.18a |
2040.8a |
426.57e |
0.0e |
3.70a |
3269.28a |
28.52c |
Table 3 Accumulation of Pb and changes in the contents of macronutrient and micronutrients in roots of cacao young plants, submitted to doses of Pb, Zn and Pb+Zn in soil to the 30 days after application of treatments. Mean values of five replicates (± SE). Letters indicate comparisons between treatments by Tukey test (p<0.05)
|
N |
P |
K |
Mg |
S |
Zn |
Mn |
Pb |
Cu |
Fe |
B |
|
|
TRAT |
-------------------dag kg-1-------------------------- |
-----------------------------mg kg-1----------------------- |
|||||||||
|
0 |
2.1a |
0.24ab |
1.27a |
0.52b |
0.15b |
63.67e |
100.9ab |
0.0c |
18.2b |
2746.6b |
24.1bc |
|
1 |
1.9ab |
0.23ab |
0.82bc |
0.44b |
0.15b |
669.5d |
97.6ab |
2089.6a |
27.3a |
2547.6b |
28.4ab |
|
2 |
1.9ab |
0.21bc |
0.63c |
0.52b |
0.15b |
1146.3c |
89.5ab |
715.1b |
25.4ab |
2972.8b |
27.3abc |
|
3 |
1.8ab |
0.25a |
0.57c |
0.44b |
0.15b |
1979.9b |
74.85b |
687.9b |
22.4ab |
2355.1b |
30.5a |
|
4 |
1.9ab |
0.22ab |
0.93b |
0.48b |
0.17a |
76.5e |
89.9ab |
3269.7a |
18.9b |
2337.7b |
27.5abc |
|
5 |
1.6ab |
0.18c |
0.33d |
1.48a |
0.12c |
2385.5a |
104.7ab |
0.0c |
22.4ab |
6401.6a |
22.4c |
Table 4 Accumulation of Pb and changes in the contents of macronutrient and micronutrients in leaves of cacao young plants, submitted to doses of Pb, Zn and Pb+Zn in soil to the 30 days after application of treatments. Mean values of five replicates (± SE). Letters indicate comparisons between treatments by Tukey test (p<0.05)
Mineral nutrients
Significant statistical differences in the accumulation of macro and micronutrients in the roots and leaves of young clonal cacao plants were found across treatments in response to increasing doses of Pb applied to the soil. However, there were no significant differences for N accumulation in the roots and leaves for all treatments (Tables 3 and 4). On the other hand, T3 (0.45 g of Zn + 0.25 g Pb kg-1 soil) and T4 (1 g Pb kg-1 soil) showed the highest P accumulations in the roots (Table 3). The same tendency was observed for the leaves; for example, T3 displayed the highest P accumulation in leaves (Table 3). Moreover, T2 (0.15 g Zn + 0.75 g Pb kg-1 soil) and T3 (0.45 g Zn + 0.25 g Pb kg-1 soil) had the highest K accumulations (1.96 and 2.4 mg dm-3 DW, respectively) in the leaves (Table 3), although these treatments also showed the lowest K contents in the roots. In contrast, T0 showed the highest accumulation of this element in the leaves (Table 4).
Only T0 and T1 differed from the others in relation to S content (Table 3). The lowest S concentrations were found in the roots (Table 3). On the other hand, the highest S accumulation in the leaves was observed mainly in T4, which had the highest dose of Pb applied to the soil (1g Pb kg-1 soil). Moreover, higher values of Ca content in the roots were observed in T3 and T4 (Table 3), while higher Ca accumulations in the leaves occurred in T1 and T2 with moderate Zn and Pb doses applied to the soil (Table 4).
Regarding Zn content in the clonal cacao plants, all treatments showed accumulation of this nutrient in the roots and leaves (Table 4). Treatment T5 (0.6 g Zn kg-1 soil) displayed the highest Zn content (2040.8 g dm-3 DW) in the roots compared with the other treatments. The same behavior occurred for Zn concentration in the leaves since this heavy metal was accumulated in all treatments, mainly in T5 (2385.5 g dm-3 DW). On the other hand, the highest Mn contents in the roots were observed in T3 and T4, while the highest leaf Mn content was found in T5, followed by T0 (Table 4).
Treatments T3, T4, and T5 showed higher Cu contents in the roots compared with T0 and T1 (Table 3). A significantly higher Cu content in the leaves was observed only in T1, which had moderate Zn and Pb doses applied to the soil (0.3 g Zn + 0.5 g Pb kg-1 soil). In addition, T5 displayed a significantly higher Fe content in the roots of young clonal cacao plants compared with the other treatments (Table 3). Likewise, a higher Fe content in the leaves was found in T5 (0.6 g Zn kg-1 soil) compared with the other treatments (Table 4). Finally, B accumulation in the roots and leaves was highest in plants grown in T3 (0.3 g Zn + 0.5 g Pb kg-1 soil) (Tables 3 and 4).
Leaf gas exchange
There were significant statistical differences for net photosynthesis per unit leaf area (A) at 15 days AAT (Figure 1A). Treatment T5 (0.6 g Zn kg-1 soil) showed a marked reduction of A, which continuously decreased leading to plant death as a result of high Zn toxicity (Figure 1A). At 0 days AAT, statistical differences in the transpiration rate (E) were found between the control and T5; this trend was maintained during the experiment (Figure 1B). However, at 30 days AAT, the plants in T3 and T5 died due to Pb and Zn toxicity. Stomatal conductance to water vapor (gs) showed the same tendency as E; specifically, the lowest gs values were found in T5 from day 0 until plant death (Figure 1C). On the other hand, at 15 days AAT, there were statistical differences for the ratio of intracellular CO2 concentration and atmospheric CO2 concentration (Ci/Ca) (Figure 1D), with the highest values observed in T5. The same results were found for intrinsic (A/gs) and instant (A/E) water-use efficiencies (Figures 1E and 1F). However, the instantaneous efficiency of carboxylation (A/Ci) presented statistical differences between T1 (0.3 g Zn 0.5 g Pb kg-1 soil) at 0 days AAT and T5 at 5 days AAT (Figure 1G). Furthermore, there were statistical differences in A/Ci between T3 and T4 (1g Pb kg-1 soil), showing the highest values, and T1, T3 and T5, with the lowest values. In contrast, by the end of the experiment at 30 days AAT, the control and T4 had the highest A/Ci values.
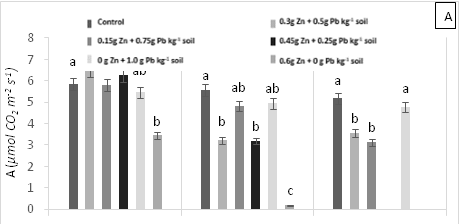
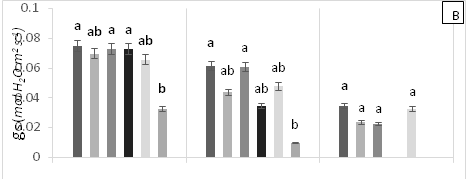
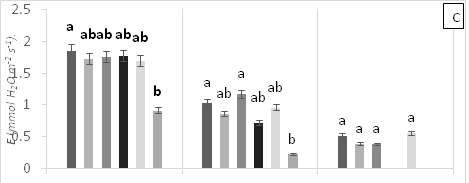
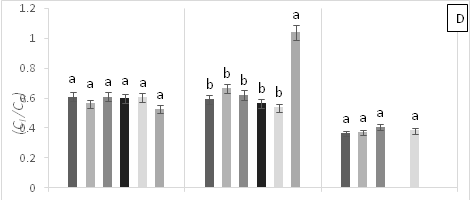
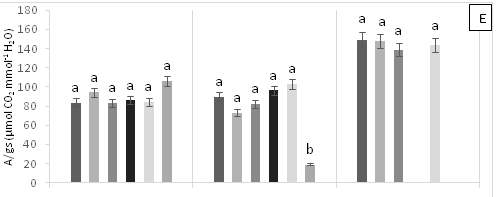

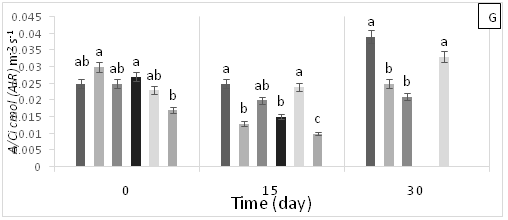
Figure 1 (A) Net photosynthesis per unit leaf area (A), (B) stomatal conductance to water vapor (gs), (C) transpiration rate, (D) ratio of internal and atmospheric CO2 concentration (Ci/Ca), (E) intrinsic efficiency of water use (A/gs), (F) instantaneous efficiency of water use (A/E), (G) instantaneous efficiency of carboxylation (A/Ci) in leaves of cacao young plants, submitted to doses of Pb, Zn and Pb+Zn in soil to the 0, 15 and 30 days after application of treatments. Mean values of four replicates (± SE). Lower case letters indicate comparisons between treatments and capital letters between time in days by Tukey test (p<0.05).
Fluorescence emission
Treatment T4 (1 g Pb kg-1 soil) showed significant statistical differences (p<0.05) at 15 days AAT for initial fluorescence (F0) (Figure 2A), demonstrating the highest values. In contrast, no significant statistical differences (p<0.05) were found for maximum fluorescence (Fm) and maximum quantum efficiency of photosystem 2 (Fv/Fm) across treatments (Figure 2B & 2C). The plants in T3 (0.45 g Zn + 0.25 g Pb kg-1 soil) and T5 (0.6 g Zn kg-1 soil) died due to the toxicity caused by high Pb and Zn contents in the soil. Therefore, there are no values for chlorophyll fluorescence emission at 30 days AAT for these treatments.
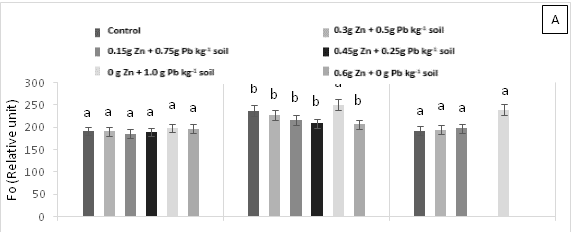
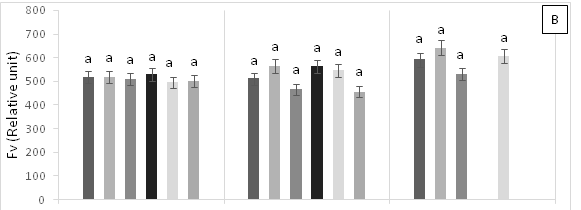
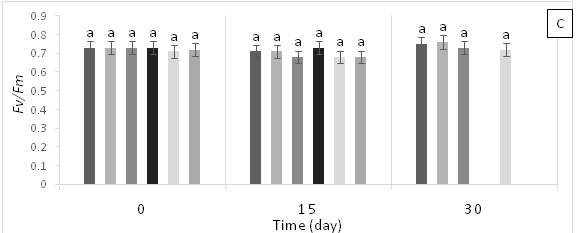
Figure 2 (A) Initial fluorescence (F0), (B) variable fluorescence (Fv) and (C) maximum quantum yield of photosystem 2 (Fv/Fm) in leaves of cacao young plants, submitted to doses of Pb, Zn and Pb+Zn in soil to the 0, 15 and 30 days after application of treatments. Mean values of four replicates (± SE). Lower case letters indicate comparisons between treatments and capital letters between time in days by Tukey test (p<0.05).
Antioxidant enzymes and proline content
Significant differences were found for APX enzyme activity in leaves of cacao clonal CCN 51 plants grown under different doses of Pb, Zn and Zn+Pb in the soil. The highest APX activities were observed at 0, 3, and 6 h AAT for T4 and at 12 h AAT for T3. In contrast, at 24 and 96 h AAT, the highest APX activity was seen in the control treatment. Furthermore, the leaves of plants in the control treatment displayed the highest APX activity in most of the time periods evaluated. The other treatments showed APX activities lower than half of those observed in the control (Figure 3A). GPX enzyme activity in leaves did not show significant changes in response to the treatments applied. In contrast, T2 showed low enzyme activity during the experiment compared with T0, T4 and T5 (Figure 3B). The plants grown in the control treatment (T0) showed low CAT enzyme activity. Moreover, CAT activity was different between the treatments evaluated. For instance, T1 showed an increase at 96 h AAT, while T2 and T3 both showed high and low enzyme activities during the evaluation period. Conversely, T4 demonstrated higher enzyme activity compared with the other treatments as the hours progressed and this activity only decreased at the end of the experiment. Finally, in T5, CAT activity gradually decreased after 3 h AAT until the end of the evaluation (Figure 3C). In plants subjected to T0 and T3, lower PPO enzyme activities were found compared with the other treatments, increasing only at 12 h AAT. On the other hand, the plants in T4 showed lower values at all evaluation hours compared with T2. Higher PPO activities were found in plants grown in T5 at 3, 6, 12 and 48 h AAT. However, at 0 h, 24 and 96 h AAT, the activities of this enzyme decreased significantly (Figure 3D). The highest SOD enzyme activities were observed in plants subjected to T4 (at 3 h AAT), T3 (at 6 h AAT), and T5 (at 96 h AAT), which showed significantly higher values than in the other treatments (Figure 3E).
The highest proline content was observed in the leaves of cacao plants in T5 at 48 and 96 h AAT, while no proline accumulation was observed in the leaves of the control treatment (T0) during the evaluation period (Figure 3F).
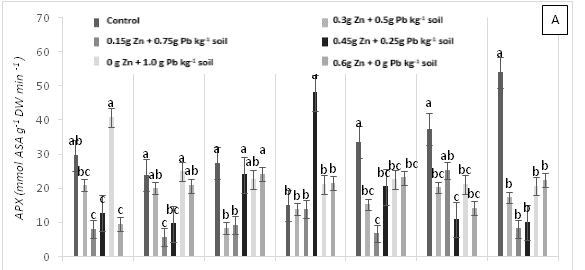
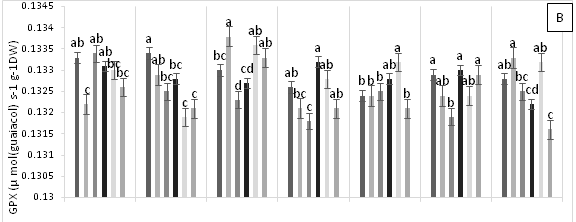
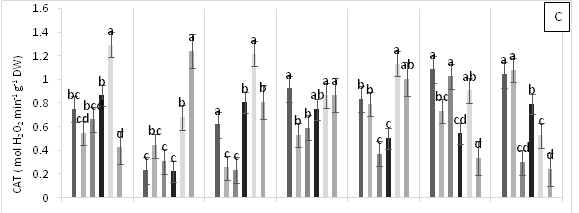
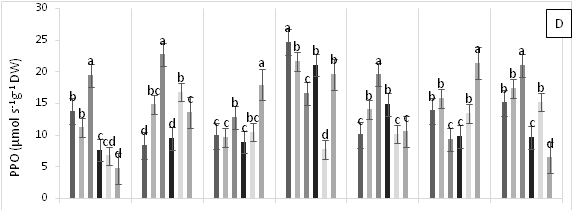
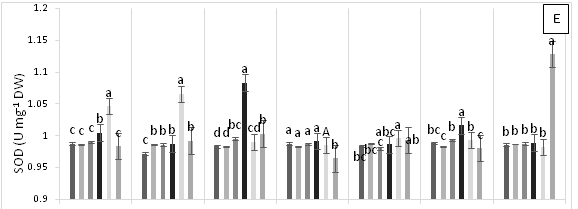
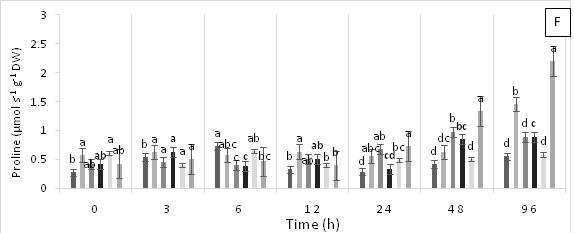
Figure 3 Enzymatic activity of APX, ascorbate peroxidase (A), GPX, guaiacol peroxidase (B), CAT, catalase (C), PPO, polyphenol oxidase (D), SOD, superoxide dismutase and proline content (F) in leaves of cacao young plants submitted to diferents doses of Pb, Zn and Pb+Zn in soil to 0, 3, 6, 12, 24, 48 and 96 h after application of treatments. Mean values of five replicates (± SE). Letters indicate comparisons between treatments by Tukey test (p<0.05).
Gene expression
There were significant differences (p<0.05) in the relative gene expression levels of psbA, psbO, Tpr1, and met in leaves in response to applications of Pb, Zn and Zn+Pb to the soil (Figure 4). The expressions of psbA at 0 h AAT were almost twice higher in T5 and T1 compared with T0 and T2. However, these expression levels were reduced by nearly half at 96 h AAT in T1 and T5 (Figure 4A). The gene expressions of psbO were higher at 0 h AAT in T3, T4 and T5. Conversely, T2 did not show expression for this gene. The highest relative gene expression of psbO was observed in T2 at 96 h AAT compared with the other treatments (Figure 4B). On the other hand, Tpr gene expressions were found in T2, T3, and T4, which showed the highest values at 0 h AAT. However, a peak in the expression of this gene was observed in T4 at 96 h AAT, with a value that exceeded the other treatments (Figure 4C). Finally, the highest expressions of met occurred at 0 h AAT for T0 and T1 and at 96 h AAT for T2, T4, and T5, with values almost twice higher compared with the control (Figure 4D).
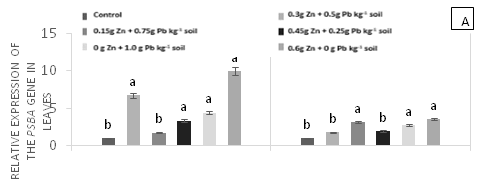

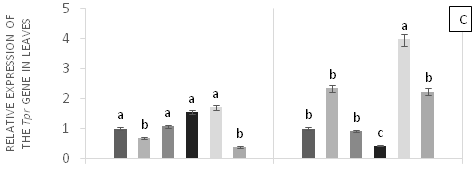
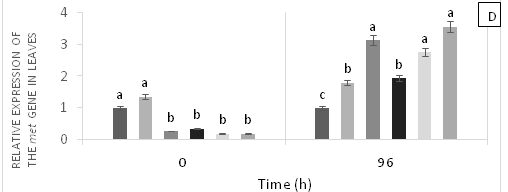
Figure 4 Relative expression of genes PsbA (A), PsbO (b), Tpr (C) and met (D) in leaves of cacao young plants, submitted to doses of Pb, Zn and Pb+Zn in soil at 0 and 96 h after application of treatments. Mean values of four replicates (± SE).The statistical significance was determined by Tukey test (p<0.05).
Many plant species will retain most of the absorbed Pb (approximately 95% or more) in the roots, while a small fraction is translocated to the aerial parts.15 Pb toxicity depends on the concentration and exposure time to this heavy metal, as well as soil physical and chemical characteristics, such as pH, cation exchange capacity, and organic matter content, which affect soil-plant absorption.25 The addition of 0.45 g Zn + 0.25 g Pb kg-1 soil and 0.6 g Zn kg-1 soil were toxic to cacao clonal CCN 51 plants. The results showed that Pb and Zn were absorbed by the root system and accumulated equally in the roots and leaves of cacao plants, demonstrating a high translocation rate for this species. A higher accumulation of metals in the roots is typical of intolerant plants, whereas the ability to translocate metals to the aerial parts is considered a tolerance factor.26
Soils containing grown plants with high Zn reserves had more nutrient availability (N, K and Cl) under normal and stressed conditions than those with lower Zn reserves, due to better root growth27,28 and a greater root surface area that increases access to nutrients. Furthermore, the availability of N was higher in soils where plants with high Zn were planted, possibly due to a positive interaction between Zn and N.29 Furthermore, higher N and Zn contents in the leaves of plants cultivated under high concentrations of Zn are possibly due to a greater exudation of organic acids from the roots, which could accelerate microbial activity and promote greater Zn solubilization28 and N mineralization. On the other hand, the positive interaction between Zn and K improves the state of exchangeable K in soil since Zn reduces leakage of amides and K, as well as maintains membrane integrity.30
Root uptake of Pb occurs through bivalent cation transporters found in the plasma membrane, such as ZIP, Zn, and Fe transporters.31 Besides, Pb can also compete for Ca+2, Mg+2, Mn+2, and Cu+2 transporters.32 In the specific case of the cacao clonal CCN 51 genotype, this competition affected the absorption of Zn and Mg and reduced Fe translocation. Zn is an essential cofactor in the oxidation pathway of the water molecule at the PS2 level.33 The higher absorption of Mn may be associated with mechanisms that reduce the toxic effects of Cd.34 On the other hand, Pb can substitute Ca+2 in calmodulin-dependent signaling pathways, such as enzymatic activation and gene expression control.35 Thus, changes in Ca content may interfere in the response of cacao plants to Pb toxicity since the translocation factor for Ca was higher in the presence of Pb. A reduced K content in cacao roots was also reported in Genipaamericana plants subjected to increasing doses of Pb in nutrient solution.36
Moreover, the data in this study demonstrated that Pb and Zn did not interfere with root and leaf contents of P and N. However, the translocation of these metals was affected since P contents increased in stems and leaves in the presence of Pb. The plants subjected to T3 (0.45 g Zn + 0.25 g Pb kg-1 soil) and T5 (0.6 g Zn kg-1 soil) died at 23 days AAT due to the toxicity of these metals at high concentrations. On the other hand, the addition of Zn to the soil increased photosynthesis in cacao young plants.However, there are reports that excess Zn may result in reduced CO2 uptake, one of the major phytotoxic effects of Zn.37 This fact was not confirmed in this study. In contrast, Pb accumulation in cacao leaves caused severe damage to the photosynthetic machinery, as previously described in the literature.32 This effect may be due to reduced expression of important genes (e.g., psbA), inactivation of enzymes involved in carboxylation, lipid peroxidation, and antioxidant defense disorder.38 Furthermore, in this research, the damages were influenced by Pb concentration and time of exposure.
The young plants of the cacao clonal CCN 51 genotype showed variations in net photosynthesis (A) after applying the treatments with Pb, Zn and Zn + Pb. In treatments containing high Zn and Pb doses in the soil, uptake of Zn and Pb by the roots and the consequent accumulation of these metals in cacao leaf dry biomass promoted reduced photosynthesis in clonal cacao plants (Figure 1). According to Moradi and Ehsanzadeh (2015), Pb accumulation in the leaves causes damage to the photosynthetic machinery, promotes alterations in PS2, and partial closure of stomata, which consequently limit CO2 assimilation. In addition, Pb toxicity can inhibit chlorophyll synthesis and disrupt the photosynthetic process.39
The Fv/Fm ratio is used as a stress indicator since it evaluates the efficiency and integrity of PS2.19 The reduced Fv/Fm in cacao leaves was caused by low Fo and Fm values.19 In a recent study that evaluated Pb toxicity in Populus plants grown in nutrient solution with increasing concentrations of the heavy metal, the authors reported low variation in Fv/Fm ratios.40 Nevertheless, the study was conducted under hydroponic conditions, the toxic metal was 100% available in solution, the pH of the solution was under control, and there was a short time of exposure to the metal.
Pb is not a redox-active metal and cannot generate ROS by direct participation in Fenton’s reactions. However, it induces ROS formation by interfering with the electron transport chain.41 ROS cause a variety of deleterious effects on plants, such as lipid peroxidation, growth retardation, chlorosis, darkening of the root system, inhibition of ATP production, and DNA damage, resulting in programmed cell death (PCD).42 To prevent ROS from irreversibly damaging the photosynthetic machinery, there are enzymatic and non-enzymatic metabolic pathways that eliminate ROS.43 In this regard, proline, which is an essential osmolyte for plants under stress, acts as an important non-enzymatic antioxidant capable of eliminating ROS produced during the stress condition and inhibiting PCD.43
Accumulation of Pb in plant tissues negatively interferes with several essential metabolic processes, promoting oxidative damage caused by ROS.43 In this sense, the increased activity of antioxidant enzymes is considered a generalized response to excess trace metals in the soil, resulting in protection against cell disturbance and damage.44 Therefore, excess Zn, as a toxic metal, can cause metabolic disturbances and macromolecule damage derived from overproduction of ROS.43
Pourrut et al.15 demonstrated that plants exposed to Pbmay increase or reduce the activity of antioxidant enzymes. In this work, the enzyme activities were lower in treatments with Pb addition to the soil compared with treatments containing Pb + Zn or in the absence of Pb (Figure 3). These findings suggest that Zn is a potent mineral that can counteract the absorption of Pb in soils with high concentrations of the latter. Thus, Zn addition to the soil can become an effective tool to reduce toxicity and prevent the negative effects of Pb on the metabolic system of the plant.
The enzymatic activities of APX, GPX, CAT, and PPO were lower when high amounts of Pbwere added to the soil (i.e., inducing stress). However, in treatments with high doses of Zn applied to the soil (T3 and T5), this heavy metal became toxic to the clonal cacao plants. Therefore, to mitigate Pb toxicity, Zn should be applied to the soil in small amounts due to its counterproductive effect.45 On the other hand, when plants are under heavy metal stress, antioxidant enzyme activities and proline content are increased.45 However, in this study, no major changes were found in the activities of enzymes involved in antioxidant metabolism in the leaves of clonal cacao plants exposed to Pb toxicity in the soil; except for SOD, which showed elevated activity at the highest doses of Zn and Pb applied to the soil. Probably, the low activity of the antioxidant enzymes may have been compensated by the increased activity of the non-enzymatic system involved in the elimination of excess ROS that were produced during Pb toxicity in plants of the cacao clonal CCN 51 genotype.
This study demonstrated that plants with high concentrations of Zn can ameliorate the adverse effects of Pb stress by reducing ROS damage through the accumulation of proline and total soluble phenolics to reduce oxidative stress. Furthermore, the superior performance of plants with high concentrations of Zn was linked to increased CCI and nutrient uptake, which improve plant growth and grain yield.46 The presence of Zn limits oxidative damage through higher accumulation of osmolytes (proline and phenolic) and reduction of total antioxidant activities and lipid peroxidation.46 However, Zn can also generate adverse effects, as observed in treatment T5 containing higher concentrations of Zn; therefore, the dosage of this micronutrient must be well regulated.
The gene expression analyses were based in the most contrasting collection time in relation to the control (0 and 96 h AAT), which was determined after evaluating the enzyme activities. At 0 h AAT, psbA showed the highest gene expression in T5 (i.e., with the highest dose of Zn applied to the soil), followed by T1. However, the activity of this gene decreased at 96 h AAT and in the control treatment (T0), which maintained stable values of gene expression during the experiment. These results demonstrate that plants subjected to variable concentrations of Zn and Pb show changes in expression of psbA gene, since these heavy metals cause oxidative stress.
Gene expression regulation in plants under stress conditions depends on the influx of Ca2+ into the cytosol, the activation of protein kinases, and protein phosphorylation. These processes have regulatory mechanisms that can be activated within seconds or minutes.47 The psbA gene is located in the chloroplast genome and encodes the D1 protein that plays a fundamental role in PS2 from the photochemical phase of photosynthesis, acting in the electron transport chain.48 The increased expression of this gene in leaves of clonal cacao plants exposed to 0.5 g Pb kg-1 soil confirms the damage caused to the quantum efficiency of PS2 due to the reduction of Fv/Fm. However, when cacao plants were exposed to 1 g Pb kg-1 soil, there was no increase in the gene expression of psbA in the leaves. Since this gene is of cytoplasmic (chloroplast) inheritance, the damage caused to this cellular organelle by Pb toxicity can reduce the transcript pool in its genome.49
Higher gene expressions of psbO were observed at 0 h AAT in T3, T4, and T5. Treatment T2 did not show significant expression, but, by the end of the experiment (96 h AAT), this treatment stood out from the other treatments. The psbO nuclear gene encodes the PsbO protein, which is extrinsic to PS2. This protein is involved in the oxygen-evolving complex and is also known as the Mn-stabilizing protein, which increases the efficiency of PS2 during the oxidation of the H2O molecule.50 Therefore, the increase in leaf Zn content in clonal cacao plants exposed to Pb toxicity in the soil may have affected the signaling pathways for psbO gene expression in this organ.51 Nascimento et al.52 found changes in the gene expression levels of sod, psbApsbO in response to the toxicity generated by heavy metals in the soil. According to this autors, the changes in gene expression altered CO2 assimilation, through decreased in gs values and enzyme activities. Furthermore, found that the highest expression levels of psbA and met occurred at higher doses of heavy metals applied to the soil at 24 and 96 h AAT. These results are similar to those found in this research since the highest expressions of these genes were observed at 96 h AAT in treatments containing the highest concentrations of Pb and Zn applied to the soil.
Since psbA is responsible for electron transfer during photosynthesis and psbO is in charge of regulating the 58psbO directly affect photosynthesis and plant growth.54 Several studies with cocoa plants suggest that the toxicity generated by heavy metals (Cd or Pb) promotes underexpression of psbO as a mechanism to protect D1 protein, which participate in oxidative stress by regulating ROS to prevent damage to PS2.55 Similar results were found in this investigation since plants subjected to higher Pb contents in soil howed altered gene expression of psbO (Figure 4b), which may be a strategy to prevent metabolic alterations caused by Pb absorption in plants.
The relative expressions of Tpr gene were high at 96 h AAT in T1 and T4 with high doses of Pb applied to the soil. Similarly, the highest expression levels of met were observed at 96 h AAT for all treatments containing Zn and Pb applied to the soil. These findings suggest that these heavy metals influenced response mechanisms of the plants to the stress. In addition, metallothioneins are proteins that also exhibit metal binding affinity, but are not involved in metal sequestration to the vacuole.56 The expression of met in cacao leaves exposed to Pb showed a similar pattern in the roots, whereas the expression of sodcyt in cacao leaves corresponded with high SOD activity in the same organ. Therefore, it is likely that the increased concentration of Fe in cacao leaves in the presence of Pb promoted the production of ROS, maintaining high expression levels of sod.57 The relative expression of met and sod genes in cocoa leaves coincided with the responses of these enzymes in the leaves of the plants evaluated. This study determined that the underexpression of these genes is related to the exposure of plants to Pb in the soil. This was also reported by Castro et al.58 when seeds from cocoa progenies derived from the self-pollination of ‘Catongo’x‘Catongo’ and of the crossing between CCN-10 x SCA-6 were immersed for 24 h in different Cd doses and by Souza et al.59 when seedlings of CCN 51 cocoa genotype were grown under greenhouse conditions and exposed to increasing Cu doses in nutrient solution.
The overexpression of genes such as met under high concentrations of heavy metals in the soil is a tolerance mechanism of Pb toxicity.60 Phytoquelatins are non-protein metal chelators that are enzymatically synthesized using glutathione as a substrate. Phytoquelatins genes are constitutively expressed, but enzymatic activation depends on the presence of the metal cofactor.61 On the other hand, metallothioneins are polypeptides that are generally synthesized under stress conditions.35 Phytoquelatins and metallothioneins act by sequestering Pb, thus, inactivating this heavy metal in the cytoplasm or transporting it to vacuoles.61 In addition, the role of phytoquelatins in the transport of heavy metal to the aerial parts of the plant has been well studied and demonstrated.62
Overall, this study demonstrated that the toxicity generated by the presence of Pb in the soil can be mitigated by the addition of Zn. The competition of these heavy metals for root absorption in cocoaplants results in lower uptake of Pb and higher availability of Zn in the soil. However, the addition of Zn can also generate toxicity in plants and cause oxidative stress, which leads to overexpression of genes such as met and Tpr (Figure 4C & 4D). On the other hand, moderate additions of Zn (0.15, 0.30 g Zn kg-1 of soil) can promote the mitigation of Pb toxicity by increasing the expression of these genes and activating all the mechanisms to stop the damages caused by oxidative stress. The presence of heavy metals in the soil considerably alters the cellular metabolism of cocoa plants, leading to death in some cases, as mentioned by Pereira et al.60
Young plants of the cacao clonal CCN 51 genotype grown in soils with high Pb, and Zn+Pb contents were found to accumulate these heavy metals in the roots and leaves.
Uptake of Pb and Zn by the roots and its transports into the aerial part promoted to significant physiological changes in the photosynthesis, nutritional balance, antioxidant metabolism, and gene expression of the young plants of the cacao clonal CCN 51 genotype.
Increased SOD enzyme activity and proline content in leaves of the young plants of the cacao clonal CCN 51 genotype contributed to mitigate Pb and Zn toxicities at the highest doses of these metalic elements applied in soil.
Zn+Pb adequate doses applied in soil mitigated the toxicity of Pb in young plants of the cacao clonal CCN 51 genotype.
Zn+Pb and Zn doses applied in soil, corresponding to 0.45 g Zn + 0.25 g Pb kg-1 soil and 0.6 g Zn kg-1 soil, respectively, induced the death of young plants of the cacao clonal CCN 51 genotype at 15 days after application of treatments.
Application of Zn in the soil can be used to mitigate the Pb toxicity in young plants of the cacao clonal CCN 51 genotype growns in contaminated soils.63–70
SENA Tecnoparque Node Yamboro and its Yamboro research group.
Authors declare no conflict of interest exists

©2020 Muñoz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.