eISSN: 2576-4462


Research Article Volume 5 Issue 5
1Environmental Microbiology Laboratory,Research Institute of Chemical Biology
2Catalysis Laboratory, Department of Chemical Engineering 3 Department of Chemical Pharmacobiology
Correspondence: JM Sánchez-Yáñez, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mich., C.P. 58060, México
Received: August 03, 2021 | Published: November 23, 2021
Citation: Castro-Villaseñor JA, Rico JL, Salcedo LI, et al. Hordeum vulgare marketed as food contains potential fungi that synthesize mycotoxin. Horticult Int J.. 2021;5(5):177-179. DOI: 10.15406/hij.2021.05.00226
The grains of H. Vulgare commonly used for the elaboration of meals for humans and animals, could be contaminated with propagules of native fungi resulted from the intensive agricultural system of production. In addition, the unsuitable storage conditions could strongly influence the growing of undesirable fungi which could potential synthetiz emyco toxins. The aim of the present research work was the isolation of Aspergillus potential to synthetize ochratoxin A from the commercially available in H. vulgare. To this purpose, the grains were collected from various local stores, from grain of H. vulgare. Aspergillus were isolated in potato dextrose and in a Sabourauddextroseagar to determine the density and diversity. The potential for the synthesis of ochratoxin A by Aspergillus strains was then evaluated. The results were statistically evaluated using the software Anova/Turkey.
Results indicate the presence of a relatively high number of propagules of Aspergillus spp which are contaminating the H. vulgare. Furthermore, 67% of the fungi present in H. vulgare have the potential to synthetize ochratoxin A. These results demonstrate the risk of consumption of those grains by humans.
Keywords: soil, native fungi, commercialization grain, food, intoxication, health
In Mexico, H. vulgareis one of the most consumed cereal by humans and animals,1 and it is cultivated through an intensive production system using pesticides and fertilizers.2 Due a this production system, the proliferation of fungi propagules such as Aspergillus spp in the soil has a consequence, the produced grains could also be contaminated by those fungy. In addition, the grain storage conditions such as humidity and temperature3 can further induced the growing of those fungi. My cotoxins are secondary metabolites which after ingestion can cause acute or chronic intoxication and damage of humans and animals. They are synthetized by some kind of fungi, such as Aspergillus, Penicillium and Fusarium. Toxins such as a flatoxins, fumonisins, trichothecenes, zearalenone and ochratoxin A are among the most important mycotoxins. The present research study is aimed to explore the possibility of finding Aspergillus in H. Vulgare collected from local stores. Moreover, the potential capacity of this genus of fungi to synthetize ochratoxin A was also evaluated.
The H. vulgare (hv) cereal was acquired from local stores from various zones in Morelia, Michoacan, Mexico. The zones were accordingly selected to the cardinal directions from north, east, west, south and central zone and the samples were labelled as N-hv, E-hv, W-hv, S-hv and C-hv, respectively. The grains were transported to the laboratory at 12oC in plastic sterilized bags. The isolation and counting of fungi was performed by dilution and using the plate counting method recommended by Pitt and Hocking, 1997. For this purpose, 1 g of hv grains was first immersed in 9 mL of an aqueous solution of 0.85 wt % of sodium chloride and 0.01 g of detergent/L. The pH was adjusted to 7. The mixture was then stirred during 30 min in a Vortex. The solution was then diluted 107 folds. Using laboratory plates, a portion of the final solution was cultivated in Sabourauddextrose agar containing (g/L) 25.0 of glucose, 10.0 of peptone, 1.0 of yeast extract, 18.0 agar per litter at pH of 5.6 to inhibit bacteria growth. The counting of fungi was then performed and it is expressed as average colony-forming units per gram, CFU/g. The representative colonies from each plate were then kept at 4oC for further studies and characterisation. The study was done in triplicate and the samples were incubated at 30oC during 48 h. By using microscopic and macroscopic reproductive characteristics of this fungy, to identify them. The synthesis of ochratoxin A by fungi was qualitatively evaluated by using a UV lamp. The extraction of this toxin from this zone was then performed taking 2.5 g of sample. Ochratoxin A was extracted using a soxhlet system and diethyl ether as a solvent at 37oC. The separated toxin was placed into Eppendorf tubes, covered with aluminium foil and kept at 0oC in the fridge prior to quantification. The concentration of ochratoxin A was determined by a spectrophotometer (VE-5000), at 350 nm.4 Various solutions with known concentrations of the ochratoxin A (>98%, Sigma-Aldrich) in ethanol were prepared to obtain the calibration curve.1 The experimental data were analysed using ANOVA/Tukey α=0.05 and the Stat Graphics Centurion software.1
Among various fungi detected in these in H. Vulgare by its reproductive characteristics, the most abundant was the genus Aspergillus spp.The potential synthesis of ochratoxin A by these fungi is therefore expected. It is documented that ochratoxin A could be produced by Penicillium verrucosum (Pitt and Hocking, 1997), and Aspergillus species, (A. ochraceus, A. alliaceus, A. carbonarius, A. niger and A. melleus. After consumption, such a toxin constitutes a risk for the human and animal health (Frisvadet al., 2007). Furthermore, ochratoxin A can be classified as a carcinogenic, hepatotoxic, nephrotoxic, teratogenic and immunosuppressive (Creppy, 1999). Table 1 exhibits the percentage of Aspergillus spp detected in H. vulgare. High values were detected in the W-hv and S-hv samples, whereas the lowest in the N-hv sample. Table 1 also shows the propagule density of Aspergillus spp determined in H. vulgare. As can be noticed, the highest density was observed in the S-hv sample with a value of 205 UFP/g and the lowest in the N-hv with a value of 49 UFP/g. The differences in the propagule of Aspergillus densities indicate the effect of the storage conditions and grain age. Independently from the acquisition zone, the cereal was contaminated with Aspergillus sp. This table also exhibits the concentration of ochratoxin A synthetized in culture media by Aspergillus spp. The highest value of 93 ppm was detected in S-hv sample and a low value of 21 ppm in N-hv. The former value is in correlation with highest propagule density of Aspergillus spp detected in H. vulgare. The high propagule density observed for C-hv and W-hv showed a low concentration of ochratoxin A which is probably due to the presence of other Aspergillus species. Other studies have reported the production of ochratoxin A in culture media from 0.01 to 234 ppm.5,6,7 The characteristic fluorescent blue hallow formed under the UV light indicates the presence of ochratoxin A8 in the fungi cultivation. The Figure 1 shows the blue hallow of a sample cultivated in Sabouraud dextrose agar.
|
*Sample |
Aspergillus sp, % |
Propagule density of Aspergillus sp, 106 UFP/g |
Ochratoxin A, (ppm) |
|
C |
0 c** |
0d** |
0 d** |
|
N-hv |
5b |
49b |
21b |
|
E-hv |
18b |
156a |
71a |
|
S-hv |
45a |
205a |
93a |
|
C-hv |
10b |
135a |
18c |
|
W-hv |
22a |
167a |
47 a |
Table 1 Propagule density of Aspergillus fumigatus in Hordeum Vulgare and the concentration of ocratoxin A synthetized by the same fungiinSabouraud dextrose agar
*equivalent to 1 kg of seeds, **values with different letters show a statistical difference, according to ANOVA-Tukey (P<0.05)
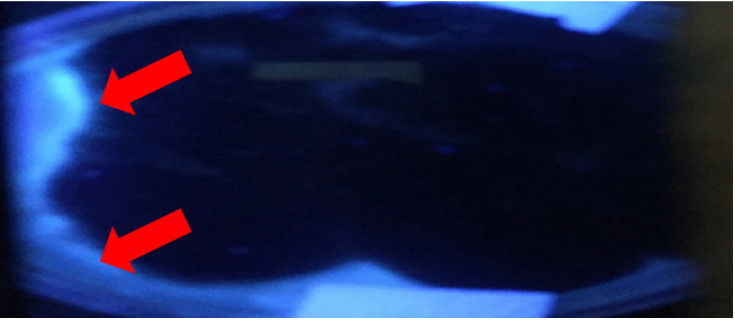
Figure 1 UV-Detection of the presence of ochratoxin A in Sabouraud dextrose agar synthetized by Aspergillus fumigatus isolated from grains of Hordeum vulgare marketed in Morelia, Michoacán, México.
Although the detection of Aspergillus spp in all samples, the differences in ocratoxine concentration suggest that various Aspergillus strain of fungi could be present in H. vulgare and that the grains come from various regions of Michoacán, Mexico.9 Other studies that found my cotoxins in various agriculture products such as coffee, grape juice, spices, wines, beer and products of animal origin were reported.5,10-15 About 25 % of the cereals consumed in the world are contaminated with mycotoxins.16 The most favourable areas for growing of fungi and therefore high probability to synthetize mycotoxins, are the regions with good weather and high humidity as Michoacán.17
The presence of propagules of Aspergillus spp found in H. vulgare, indicates the high probability for the synthesis of ochratoxin A. This toxin represents a high health risk for humans and animal after its consumption as well as the transportation, marketing conditions for H. vulgare in the city of Morelia, Mich, México in order to avoid the production synthesis of ochratoxin A should be important for the corresponding sanitary authority.
The authors thank CIC-UMSNH Proyecto 2.7 (2020) and BIONUTRA S.A Maravatio, Mich, Mexico for the financial support to perform this research project.

©2021 Castro-Villaseñor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.