eISSN: 2576-4462


Research Article Volume 2 Issue 6
Biotechnologies and Valorization of Natural Resources (LBVRN) Faculty of Sciences, University Ibn Zohr, Morocco
Correspondence: El Madidi Said, Biometrics and Bio Resources. Laboratory Biotechnologies and Valorization of Natural Resources (LBVRN) Faculty of Sciences, University Ibn Zohr, Agadir, Morocco
Received: January 01, 1971 | Published: December 13, 2018
Citation: Said ElM, Fatiha H. Genotypic variability in fruits characters of moroccan watermelon cultivars ( Citrullus Lanatus ) cultivars under well and limited watered conditions. Horticult Int J. 2018;2(6):378-381. DOI: 10.15406/hij.2018.02.00080
The aim of this study was to determine the effects of water stress on 9 watermelon genotypes among which five watermelon landraces collected in two localities (rasmouka and zagoura) and four commercial varieties F1. Two irrigation levels were imposed to determine variability on drought tolerance of cultivars. After the true leaves, irrigation treatments were initiated. T1 treatment received full irrigation based on depleted soil moisture in the root zone, T2 treatment received 50 % of T1.The drought tolerance was estimated by the ratio of the value of a trait under the T1 irrigation level and the value of this trait under the T2 irrigation level. For all the characters the mean values recorded in well irrigated conditions were greater than recorded in limited irrigated conditions. The analysis of variance revealed that genotypic differences were highly significant for all characters. Genotypes x trials interactions were also highly significant for all characters except TTS. Moderate to high values of broad-sense heritability were estimates for all characters measured except for fruit rind thickness. The values of heritability in limited watered where lower to those obtained in well‐watered conditions and the TSS had the highest value of heritability in well and limited watered conditions. The results show that the modern varieties are more susceptible to the hydric stress than the local landraces and suggest that genotypic variability for tolerance to drought stress exists among Moroccan watermelon cultivars and there is a need to screen a large number of genotypes to identifying and selection drought-tolerant genotypes.
Watermelon (Citrullus lanatus (Thunb.)Matsum.&Nakai) is an important vegetable fruit crop for human consumption. Over the years, watermelon production has increased steadily. According to the Food and Agricultural Organization of the United Nations (FAO), The world area harvested is 3477469 ha and the annual world production of watermelon is about 111 million tons in 2014 (FAO, 2017). In Morocco, the area harvested is 15969 ha and the annual production of watermelon is about 724915 tons in 2014 (FAO, 2017).Watermelons belong to the xerophytic genus Citrullus Schrad are cultivated in temperate and tropical regions of the world, serving as a water and food source for animals and humans.1,2 Drought is the most important environmental stress for agriculture and many efforts have been made to improve crop productivity under water-limited conditions.3–5 Dessert modern watermelon has a narrow genetic base.6–8 Landraces can provide importants traits for tolerance to drought and heat, such as higher biomass, which would greatly improve the crop’s adaptation to climate change worldwide.9 Identification of watermelon germplasm with drought tolerance properties is needed for the development of drought tolerant watermelon varieties. The agricultural use of water is higher than 85% in the Souss Massa region of Morocco, resulting in an increasing water deficit in the region. There is therefore a need for study of genotypic variation in cultivated watermelon genotypes and evaluation for tolerance to limited irrigated conditions.
Two field experiments were conducted at Sidi bibi Training Research Station (30° 11' 18,3" N, 9° 32' 17,6" W) during the growing seasons 2014-2015. 9 watermelon cultivars: Five watermelon landraces (RM1, RM2, RM3, ZG1, ZG2) and four commercial varieties F1 (Cerrato, Venizia, Daytona and Farao) were used in this study. Watermelon landraces are native from two arid regions of Morocco (rasmouka and zagoura). The samples of landraces were collected from farms wich had produced their own seed for at least 10 years and then were multiplied and homogenized during the growing seasons 2013-2014. Two irrigation levels (Table 1) were imposed to determine variability on drought tolerance of cultivars. T1 treatment (Well irrigated) received full irrigation which plants received sufficient water to maintain soil water content close to pot capacity, While T2 (limited irrigated) treatment received 50 % of T1. Irrigation water was applied via a drip irrigation system with flow rate of 2 l h-1. The irrigation was controlled manually using the valve on each manifold. Plots were irrigated for the period of calculated time. Irrigation quantities for plots was recorded and used for the calculation of the total irrigation amounts. The following characters were measured: fruit weight (FW), fruit length (FL), fruit width (FWd), fruit rind thickness (FRT) and total soluble solids content (TSS). Each treatment was set up in a randomized block design with three replicates, Every elementary plot includes 10 plants spaced out by 0,8 m of inter-plants and 3,5 m of line spacing. For each experiment, data were measured in five plants randomly selected and ten fruits per plant. A total of 30 fruits were scored for each cultivar in each experiment. For each character, the percentage of reduction was calculated to evaluate the response to water stress, according to the following formula: % of reduction = (1-(y/x)) x100. Where x and y are the mean values of the examined character in stressed and non stressed experiments. All ratios were arcsine transformed and analysed in two-way analysis of variance. Differences between the means were compared using the Newman-Keuls Test. The phenotypic variance in each trial: . The Broad-sense heritability was estimated by: . The expected genotypic advance (genotypic gain) was estimated by: where i= selection differential, the value is 1.40 at 20% selection intensity. The relative genotypic gain is obtained by dividing the geno typic gain by the mean of the character measured (as percent of mean). Statistics analysis was carried out using computer software SAS version 9.3 (SAS Institute Inc. 2010) (Table 1).
|
Precipitation (mm) |
Irrigation (mm) |
Total (mm) |
Trial 1(WI) |
82 |
566 |
648 |
Trial 2(LI) |
32 |
283 |
315 |
Table 1 Hydrique conditions in the two field experiments
WI, well irrigated; LI, limited irrigated
Descriptive Statistics of fruit characters analyzed are presented in Table 2. The coefficient of variation (CV) estimate ranged from 17% to 43% in WI and from 17% and 54% in LI. Mean fruit weight values ranged from 1.07 to 15,72Kg in WI and from 0,99 to 10,91 in LI. For all the characters the mean values recorded in well irrigated conditions were greater than recorded in limited irrigated conditions.
|
Trial |
Mean |
Min |
Max |
SD |
CVP |
FW(kg) |
1 |
5.69 |
1.07 |
15.72 |
2.44 |
43% |
2 |
4,05 |
0,99 |
10,91 |
2,18 |
54% |
|
FL(cm) |
1 |
27.37 |
12 |
46 |
5.55 |
20% |
2 |
24, 36 |
12,5 |
38,90 |
5,31 |
28% |
|
FWd(cm) |
1 |
19.81 |
12 |
32 |
3.34 |
17% |
2 |
17,87 |
10,5 |
25,3 |
3,30 |
18% |
|
FRT(mm) |
1 |
16.41 |
6.43 |
36.01 |
4.23 |
26% |
2 |
12,95 |
6 |
27,5 |
3,34 |
32% |
|
TSS(°Brix) |
1 |
8.42 |
2.4 |
12.8 |
1.74 |
21% |
|
2 |
7,34 |
4,4 |
11,7 |
1,45 |
17% |
Table 2 Descriptive Statistics of fruit characters analyzed
WI , (well irrigated); LI ( limited irrigated)
The analysis of variance revealed that differences between the two irrigation levels (the effect of irrigation) were highly significant for all characters except TTS. The effect of cultivars was highly significant for all the traits studied. This indicates the existence of a high degree of genotypic variability in the material studied. Genotypes x trials interactions were highly significant for fruit weight, fruit length, fruit rind thickness, and significant for TSS, indicating that differences among mean values of cultivars varied with irrigated conditions (Table 3).
SV |
DF |
FW |
FL |
FWd FRT TSS |
|
Exp |
1 |
51,61 |
45,35 |
41,49 23,82 1,69 |
|
*** |
*** |
*** *** ns |
|||
GEN |
8 |
6,19 |
7,67 |
3,57 4,18 2,84 |
|
*** |
*** |
** ** ** |
|||
Exp x Gen |
8 |
10,99 |
7,27 |
11,83 3,12 2,07 |
|
*** |
*** |
*** ** * |
|||
Error |
522 |
||||
Total |
539 |
|
|
|
|
Table 3 Results of analysis of variance (Fisher-Snedecor values)
FW, fruit weight; FL, fruit length; FWd, fruit widt; FRT, fruit rind thickness; TSS, total soluble solids content. DF, degree of freedom ***, **, *: significant level at 0.001, 0.01 and 0.05 respectively
In Table 4, the mean values in the well irrigated and limited irrigated experiment, the percentage of reduction are reported for each character. In the limited irrigated experiment lower values were found for all the characters. The percentage of reduction ranged from 1% and 29% recorded on TSS and FW respectively.
Character |
Trial 1 (WI) X |
Trial 2 (LI) Y |
% of reduction (1- (Y / X)) x 100 |
FW |
5,69±2,44 |
4,05± 2,22 |
29% |
FL |
27,37±5,55 |
24,37±5,31 |
11% |
FWd |
19,80±3,34 |
17,87±3,30 |
10% |
FRT |
16,40± 4,23 |
12,95±4,92 |
21% |
TSS |
8,42±1,74 |
8,35±1,45 |
1% |
Table4 Mean and percentage of reduction for each character analysed
In Table 5, the percentage of reduction (PR) and the rank are reported for each genotype. The ranking order was different; the ranking order of the landrace RM1 was the first in FW, FL, FWd and the second in FRT and TSS. The ranking order of FAR was 7th in F, FL, FRT, TSS and the 8th in FWd. The mean ranking order ranged from 1, 4 and 7, 2 recorded respectively in RM1 and FAR. The landraces RM1, ZG1 and ZG2 are the lowest mean ranking order while the highest values were recorded in the commercial variety FAR and VEN. The productivity of watermelon are frequently limited by various biotic and abiotic stress factors, such as drought, salinity, high and low temperatures, nutrient deficiencies, disease, and insect pests. Drought stress can affect yield through different mechanisms across the whole life cycle of the watermelon plant.10,11 The domestication and intensive selection for desirable fruit quality resulted in a genetic bottleneck and a narrow genetic base among watermelon cultivars.6,12 The narrow genetic base of dessert watermelon (Citrullus lanatus) cultivars creates a continuous challenge for researchers and breeders aiming to improve the crop for biotic and abiotic tolerance. Plant landraces represent heterogeneous local adaptation of domesticated crops and might be expected to be useful sources for enhancing tolerance to biotic and abiotic stresses Table 6 & Figure 1.
|
FW |
FL |
FWd |
FRT |
TSS |
Mean range |
|||||
PR |
R |
PR |
R |
PR |
R |
PR |
R |
PR |
R |
||
RM1 |
-0,13 |
1 |
-0,09 |
1 |
-0,04 |
1 |
0,06 |
2 |
-0,05 |
2 |
1,4 |
RM2 |
0,5 |
5 |
0,19 |
5 |
0,16 |
5 |
0,06 |
3 |
0 |
4 |
4,4 |
ZG1 |
0,09 |
3 |
0,06 |
2 |
-0,04 |
2 |
-0,09 |
1 |
-0,06 |
1 |
1,8 |
ZG2 |
0,04 |
2 |
0,06 |
3 |
-0,01 |
3 |
0,19 |
5 |
0,02 |
5 |
3,6 |
CER |
0,66 |
8 |
0,31 |
8 |
0,28 |
7 |
0,19 |
4 |
0,04 |
6 |
6,6 |
DAY |
0,46 |
4 |
0,19 |
4 |
0,12 |
4 |
0,23 |
6 |
-0,02 |
3 |
4,2 |
FAR |
0,64 |
7 |
0,28 |
7 |
0,33 |
8 |
0,28 |
7 |
0,08 |
7 |
7,2 |
VEN |
0,6 |
6 |
0,23 |
6 |
0,2 |
6 |
0,31 |
8 |
0,19 |
8 |
6,8 |
Table 5 Percentage of reduction and ranking order of different cultivars
|
Character |
H2 (WI) |
H2 (LI) |
R∆Gi (WI) |
R∆Gi (LI) |
|
FW |
0.41 |
0.39 |
24.68% |
19. 45% |
|
FL |
0.45 |
0.33 |
12.60% |
9.33 % |
|
FWd |
0.43 |
0.26 |
10.23% |
7.21 % |
|
FRT |
0.13 |
0.09 |
4.73% |
2.71 % |
|
TSS |
0.62 |
0.53 |
18.23 |
13.47% |
Table 6 The Broad-sense heritability and relative genotypic gain
WI, well irrigated; LI, limited irrigated
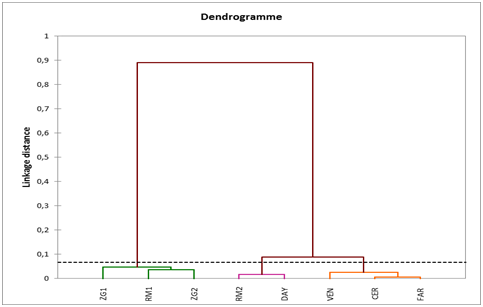
Figure 1 Dendogram of cluster analysis of the 9 genotypes classified according of the percentage of reduction.
The heritability in the broad sense is defined as the proportion of phenotypic variance that is attributable to an effect for the whole genotype, comprising the sum of additive, dominance, and epistatic effects.13,14 Heritability is a key parameter in quantitative genetics because it determines the response to selection. The values of heritability and relative genotypic gain under stress where lower to those obtained in well‐watered conditions (Table 6). The broad sense heritability estimates for fruit weight, fruit length and fruit width ranged from 0.41 to 0.45 in (WI) and from 0.26 and 0.39 in (LI), indicates large environmental effect. Gusmini and Wehner,15 reported that broad-sense and narrow-sense heritability estimates for fruit weight were low to intermediate (0.59 and 0.41, respectively) and a high number of effective factors (mean, 5.4) was found to influence this fruit character in watermelon. For total soluble solids content (TTS), high heritability is observed in this study. Similar results was also reported in watermelon16–18 suggesting that genotypic components may play an important role in the improvement of this trait in watermelon and genetic advance could be effectively used in selection on the basis of phenotypic performance. Selection for drought tolerance is one way of reducing the impacts of water deficit on crop yield. Direct selection under stress has been considered ineffective, because the broad-sense heritability (H) under stress is assumed to be lower than in non-stress environements.19–21 However, other researchers suggest that direct selection under drought stress can produce yield gains without reducing yield potential and selection for yield under stress is the most effective approach to identifying drought-tolerant genotypes combining high yield potential with high levels of drought tolerance.21–23
This work is supported financially by the University Ibn Zohr. We are grateful to DPA (Zagoura) and farmers in Rasmouka for their substantial help in collecting the watermelon landraces.
The author declares there is no conflicts of interest.

©2018 Said, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.