eISSN: 2576-4462


Mini Review Volume 5 Issue 1
Postgraduate and Investigation, InstitutoTecnológico Superior de Zacapoaxtla, México
Correspondence: Citlalli Harris-Valle, Postgraduate and Investigation, InstitutoTecnológico Superior de Zacapoaxtla,Carretera Acuaco-Zacapoaxtla Km. 8, Col. Totoltepec, 73680 Zacapoaxtla, Puebla, México, Tel 55 2333175000
Received: January 05, 2021 | Published: February 1, 2021
Citation: Harris-Valle C, Ramírez-Morales M, Mora-Guzmán E, et al. Fungi diversity associated to root of wild orchid species from zacapoaxtla and xochiapulco in sierra nororiental poblana, Mexico. Horticult Int J. 2021;5(1):30-33. DOI: 10.15406/hij.2021.05.00199
In this work are described endophytic fungi associated with orchids, since they can have the function of facilitating the nutrients assimilation or act as biological barriers against infections in plants, although some fungi are of the parasitic type. The fungal species associated with different native orchid species of the Sierra Nororiental Poblanain Mexico were isolated and identified to know the diversity of them,and infer about the importance of the plant-fungus interactions. Fungi could only be identified up to genus, one isolated species is symbiont, the most isolates are pathogens and secondly the number of antagonist isolates. The purpose is to be able to obtain one or more fungal isolates that facilitate the propagation of these important ornamental species.
Keywords: orchid, mycorrhiza, antagonist, pathogen
Orchidaceae family is a group of plants with high demand in the market for its ornamental use; it has morphological characteristics that have placed them among the most sought-after collectible organisms.1 In the Northeast Region of Puebla, Mexico, there is a wide diversity of orchids. Pérez-Bravo et al.2 describe species of orchids that are distributed in a restricted way in the Sierra Madre Oriental.
However, the continuous extraction of specimens in some regions of Mexico, for ornamental or ritual purposes, has significantly reduced the wild populations of this group of plants.3 This plants reproduction is complicated, due to the fertilization probability in the flowering season, the germination rate of the seeds and the survival of the seedlings.4,5 Thus, the cultivation difficulty means that a large number of wild specimens are illegally extracted for wild forest.
It is known that a strategy to increase the reproduction and survival of cultivated orchids is the establishment of interactions with fungi through the roots; it is also proposed that there has been coevolution between some of the associated fungi and the plants of this family.6,7 Fungi improve plant growth in various ways, by increasing their germination rate and decreasing the development time of the foliar system.8 The endophytic fungi can act as biological barriers, avoiding parasitism in the roots.9 However, some endophytic fungi are classified as pathogens under certain circumstances, that is to say, they do not always cause orchid mortality.10 The percentage of fungal colonization in orchid roots varies according to the root type.11 Rivas et al.12 found that roots linked to the substrate always show fungal colonization, the frequency being higher in terrestrial orchids.
This study have the propose to know the variations in diversity of fungi associated with the roots of wild plants, increasing the information about the ecophysiology of the Orquidiaceae family and aims to lay the foundations for the development of a plant propagation strategy for commercial purposes, thus reducing pressure on wild populations.
The plants were sampled in the localities of Apulco in the municipality of Zacapoaxtla and La Manzanilla in the municipality of Xochiapulco, in the Northeast Sierra of the State of Puebla, Mexico, at two seasons (autumn and winter). A photographic record was made of each plant for its identification, and root samples were taken.
To isolate the endophytic fungi, the roots velamen was removed under a stereoscope in a sterile environment. Subsequently, they were placed in 70% ethanol for one minute and 3% sodium hypochlorite for 30 seconds, washed three times with sterile distilled water. The fragments were placed on PDA medium (potato, dextrose and agar) and were cultivated in the dark at 25 ° C in an incubator; the colonies were isolated by successive culture.
The description and identification was considering the texture, pattern and coloration of the colonies, as well as the presence of sclerotia. At the microscopic level, was verified the branching angles, constrictions and septa at the branching points, the presence of sporangia or conidia, moniloid cells and the number of nuclei in the young fungi cells, also it was observed when mycelium was staining with safranin. Taxonomic keys and images were used to identify the fungi to genus, based on description published by Barnett and Hunter13 and the “Dichotomous key for the identification of systematically isolated fungi in Mediterranean environments” by Girmé et al.14
Orchid species found in Apulco (Zacapoaxtla) were epiphytic, only one was terrestrial; in La Manzanilla no epiphytic species was found. Of the plants species found, four were not identified and are mentioned as unidentified (ND). The wild plants had a vigorous appearance, however only in the autumn flowering were observed in most of the plants; and in winter only Epidendrumtuxtlensis was in bloom.
The maximum number of fungal isolates from the specimens sampled is 4, Trichoderma were the most common associated genus. Only one mycorrhizal fungus was found (Rhizoctonia sp.) in Stanhopea sp. (Table 1).
Orchids specie |
Autumn |
Winter |
Lycaste sp. |
Trichoderma sp. 1 |
- |
Trichoderma sp. 2 |
||
Dichaeaglauca |
Not determined1 |
- |
Penicilliun sp. |
||
Isochilus sp. |
Not determined 2 |
- |
Verticillium sp. |
||
Not determined 3 |
||
Stanhopea sp. |
Penicillium sp. |
Penicillium sp |
Rhizoctoniasp .1 |
||
Trichoderma sp. |
||
Mucor sp. |
||
Epidendrumtuxtlensis |
Trichoderma sp. |
Aureobasidium sp. |
Rhizopus sp. |
||
Acremonium sp. |
||
Pleurothallis sp. |
Verticillium sp. |
ND |
Trichoderma sp. |
Acremonium sp. |
|
Trichoderma sp. |
||
Govenia sp. |
Peniicillium sp. |
- |
Not determined 4 |
||
Prosthecheamichoacana |
Trichoderma sp. |
Penicillium sp. |
Stachybotrys sp. |
||
Verticillium sp. |
||
Prosthecheavitellina |
Cladosporium sp. |
- |
Penicillium sp. 1 |
||
Penicillium sp. 2 |
||
Not determined 5 |
||
Bletia sp. |
- |
Mucor sp. 1 |
Mucor sp. 2 |
||
Stellis sp. |
- |
- |
Maxillaria sp. |
- |
Acrmonium sp. |
Malaxis sp. |
- |
- |
Prosthecheamichuacana |
- |
- |
Isochilus sp. |
- |
- |
Coelia sp. |
- |
Trichodermasp. |
No identificada |
||
Trichodermasp. |
||
ND 1 |
Aureobasidium sp. |
Phytophthora sp. |
Cladosporium sp. |
||
Penicillium sp. |
||
Botrytis sp. |
||
ND 2 |
Penicillium sp. |
- |
Trichoderma sp. |
||
ND 3 |
Geotrichum sp. |
- |
ND 4 |
Acremonium sp. |
- |
Table 1 Fungi isolated from the roots of orchid species sampled in Apulco and La Manzanilla in autumn and winter. Unidentified plant species are named with the initials ND (no determinate), endophytic fungi species unidentified are pointed out as Not determined
In one species of orchid was not possible to isolate any fungus from the collected roots, in the same way the fungal isolates are different depending on the season.
Classification was made according to the general habits of each isolated endophytic fungus (symbiont, antagonist or pathogen). Figure 1 indicates that the endophytic fungi more common associated, during two seasons,are those classified as pathogens. Antagonists are in second place and were more abundant in autumn than in winter. The only one symbioticfungy was isolated from orchid roots collected in autumn.
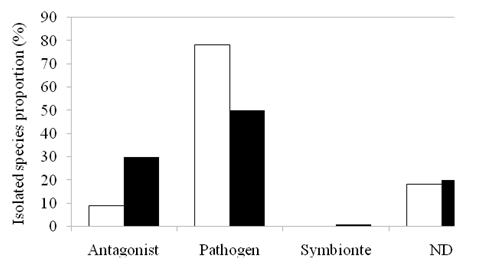
Figure 1 Proportion of isolated fungi classified according to their habits in Apulco and Manzanilla during autumn (white bars) and winter (black bars).
It should be noted that the proportion of pathogens in winter is 20% higher than in autumn, while the opposite is observed in the proportion of antagonists (33 and 10% in autumn and winter, respectively).
One factor that determines the habit of orchids (epiphytes or terrestrial) is the intensity of light. Terrestrial plants are much more sensitive to light, mainly during germination, and altitude also directly influences the floristic distribution within the ecosystems where these species live.15 It is also considered that the distribution of terrestrial orchids depends on different environmental factors such as altitude;16 in this study there is a higher altitude in the municipality of Xochiapulco, where all the orchids found were terrestrial.
It was found that the presence of endophytic fungi varies according to the season in the entire orchid species studied, in the same way Rassmussen and Whigham17 observed a change in Galearisspectabilis when is compared spring and autumn.
Several of the endophytic fungal genera isolated from the orchids, in both locations, have already been reported by other authors. For example, species of the genus Penicillium and Fusarium are endophytic fungi in epiphytic orchids according to Sudheep and Sridhar.18
In general, it is considered that the specificity is a function of the plant species and the stage of development of the plant,19,20 as well as the growth habit,21 which coincides with results described for the orchid species sampled in this work. Although most fungal organisms isolated from roots can be broadly classified as pathogens, some are not necessarily harmful to orchids. Angel et al.22 found that Fusarium can promote growth in some species by acting as an endophyte while for others, such as the catleyas, it behaves as a pathogen that causes anthracnose. The genus Trichoderma, was one of the most frequent fungi in the sampled plants, is classified as an antagonist and has the ability to create a favorable environment to radical development, which increases the tolerance of plants to stress and acts as a biological biocontroller.23
Endophytic fungal variations associates with wild orchids confirm that the fungus-plant relation ships in the Orchidfamily are spatially and temporallycomplex, so the design of strategies in which fungi are used that facilitate the individuals germination and establishment, under controlled conditions, requires ecophysiological that allow the selection of effective endophytic fungal species for culture certain plants species depending of environmental conditions. We consider that the symbiont species (Rhizoctoniasp.) and the antagonist fungus (Trichodermasp.) are candidates to evaluate their functionality in germination increasing, plant survival and establishment of cultivated orchids.
Wethank Mr. Sadot Mora Ortigoza for his support and collaboration throughout the development of the experimental work. As well as the Tecnológico Nacional de México for the project financial support of the proyect: "Establishment of Orchid Garden for the propagation of plants in association with native endophytic fungi of the Sierra Nororiental Poblana" (Code 5065.19-P) during 2019 within the Research Support Scientific and Technological in the Educational Programs of the Decentralized Federal Technological and Centers.
Authors declare no conflict of interest.

©2021 Harris-Valle, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.