eISSN: 2576-4462


Research Article Volume 5 Issue 1
1Institute of Genetic Resources and Productivity, Colegio de Postgraduados, Mexico
2Institute of Natural Resources, Colegio de Postgraduados, Mexico
3Institute of Socio-Economics and Computer Science of the Postgraduate College, Mexico
Correspondence: Agustín Damián Nava, Institute of Socio Economics and Computer Science of the Postgraduate College, Universidad Autonoma de Guerrero, Km. 36.5 Mexico-Texcoco highway CP 56230, Montecillo, Edo. from Mexico
Received: February 03, 2021 | Published: February 18, 2021
Citation: Alvarez DV, Hernández MS, Hernández VAG, et al. Flavonoids in Psidium guajava L. leaves. Horticult Int J. 2021;5(1):38-41. DOI: 10.15406/hij.2021.05.00201
Introduction: The leaves have been extensively investigated in the biology activity in human and animal health, where important metabolites such as phenols have been associated, of which the following stand out: flavonoids, tannins among other compounds, however this document aims to locate the anatomical location and analyze the content of major flavonoids in the mature and immature guava leaf.
The study was complemented with the analysis of the ethyl ether and butanol extracts; The two types of leaves with their respective extracts were analyzed by thin layer chromatography, which allowed them to be detected and their concentration estimated by liquid chromatography.
The study indicated that the mature leaf shows a large amount of flavonoids located in the adaxial and abaxial epidermis, as well as in glands, while in the immature leaf in the adaxial epidermis and in the abaxial epidermis it only presented a minimal proportion of them. Conclusion: Total flavonoid concentrations range from 2610 mg *kg-1 in the mature leaf to 2016 mg* kg-1 for the immature leaf, quercetin being the one that was found and observed in the highest proportion in both leaves.
Keywords: flavanols, flavones, guava, fluorescence, leaves, chromatography
Guava is one of the most important crops for being a source of vitamin C, and rich in flavonoids.1 In different countries and in different empirical preparations such as infusions, guava is used to cure more than 40 diseases. Traditional medicine in Mexico indicates guava leaf infusions to relieve abdominal pain associated with acute diarrhea, in addition to reducing the frequency of bowel movements. In the case of flavonoids, there is evidence of their functions in the plant,2 such as self-defense,3 resistance to drought4 and UV light protection.5,6 The location of flavonoids has been identified in different genetic materials and in different organs such as leaves,7 petals,8 trichomes,9 root, in meristematic and mature cells,9 epidermis.10 The location of this material in some cases was detected by its autofluorescence and in others by a fluorochrome (NP).7 There is little information on the anatomical location of these compounds in guava organs and their behavior at different stages of development. The purpose of this work is to identify and quantify some of the types of flavonoids and their anatomical location in the immature and mature leaf of P. Guajava.
Immature and mature leaves were collected from Psidium guajava trees of the seven-year-old cultivar media china, under greenhouse conditions.
The anatomical location of the flavonoids was made with a fluorescence microscope based on the method of Markham et al.7
On fresh materials, 30 micron cross sections were made with a handheld microtome. Some sections were immersed intact in water at room temperature. Other sections were immersed in a saturated solution of Naturstof reagent (NP) with 10% sucrose for two hours.
Subsequently, the sections were mounted in the same solution in which they were treated and were observed in an inverted fluorescence microscope with filter number one of 490-520nm.
Aglycons and glycosides were identified with a histochemical test. For this, intact sections of the two types of leaves were taken and immersed in methanol, ethyl ether and n-butanol.
For the previous identification of flavonoids in thin layer chromatography, the extracts were obtained as follows: 100 g of material from both leaves was extracted with methanol for 72 hours with three changes of solvent. The final volume was concentrated under reduced pressure. The solid extract was resuspended in water and in a liquid-liquid extractor it was extracted with petroleum ether to extract waxes and lipids, once the extraction was exhausted, the petroleum ether was exchanged for ethyl ether and later when this extract was exhausted it was changed for n - butanol.
The ether extracts of immature and mature leaves, as well as the butanolic extracts, were analyzed by silica gel thin layer chromatography with ethyl acetate: formic acid: acetic acid: water (100: 11: 11: 26).
To confirm the presence of flavonoids, the extracts were analyzed by HPLC based on the method of Crozier et al.11 and in particular there were three flavanols: myricetin quercetin and kaempferol and two flavones luteolin and apigenin, to which the greatest attention was paid.
For the HPLC analysis, the extracts were concentrated in vacuo and weighed to obtain the yield and later 0.25 g of crude extract were taken and 25 mL of 1.2 M HCl in 50% aqueous methanol were added. From there an aliquot of 50mL and titrated to 250 mL with acidified water (pH 2.5 with trifluoroacetic acid) and analyzed by HPLC (Agilent Technologies chromatograph) with diode array detector, a hypersil ODS column of 125 mm long and 4 mm internal diameter and 5 micron . At a wavelength of 365nm; with a flow of 1.5 mLmin-1 with a 65-35 isocratic gradient with methanol-water, the water acidified to pH 2.5 with trifluoroacetic acid.
Descriptive analysis of cross sections of mature and immature guava leaf by means of fluorescence microscope plus the specific fluorochrome for flavonoids (NP); showed images that are presented below: figure one shows the immature leaf with yellow-orange fluorescence in the adaxial epidermis and blue fluorescence in the abaxial; and in mature leaves in both epidermis, these results coincide with Markham et al.7 Ormrod et al.12 Olsson et al.13 Buchholz et al.14 who mention that flavonoids are found in this anatomical part and show the characteristic yellow-orange fluorescence. The glands of both leaves showed yellow orange fluorescence. The glands can be found in both the epidermis and hipodermis (Figure 1) (Figure 2), The anatomical sections of the immature leaf showed fluorescence in the epidermis of the bundle (yellow-orange) and underside (blue), gland (G) on the underside (EE), mesophyll (PE and PE) and vascular bundle (HV). The hypodermis (H) does not show fluorescence (Table 1).
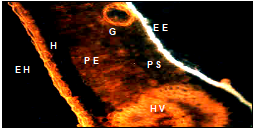
Figure 1 Anatomical section of an immature leaf shows fluorescence in the epidermis of the bundle (EH), underside (EE), gland (G) spongy parenchyma (PS), palisade (PE), hypodermis (H) and vascular bundle (HV).
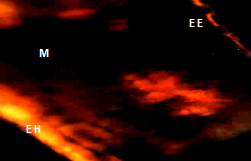
Figure 2 Anatomical section of a mature leaf shows fluorescence in the epidermis of the bundle, underside, spongy parenchyma gland, and palisade and vascular bundle.
Solvent |
Fluorescence h. immature underside beam |
Fluorescence h. mature underside beam |
||
Water |
blue |
Orange nd |
Orange nd |
Orange nd |
Methanol + NP |
nd |
Orange |
Orange |
Orange |
Ethyl ether + NP |
Nd blue |
Orange |
Orange |
Orange |
n-butanol + NP |
||||
Table 1 Fluorescence of anatomical sections of guava (hi = immature leaf and hm = mature leaf) after extraction with solvents
NP =- diphenyl boric acid ethyl amino ester; na = not detected
In order to corroborate the previous test, a histochemical test was carried out in which the sections were placed in water plus the fluorochrome, said section showed fluorescence in glands, and in the two epidermis of mature and immature leaf, which indicates the presence of the flavonoids at these sites; the colors orange are assumed to be flavones and flavanols and blue can be phenolic acids and some tannins as recently mentioned by Elixabet et al.15 and Weimin et al.16
The sections were also subjected to a solution with methanol plus fluorochrome, said solvent is recommended to extract flavonoids. The image of this treated section did not show fluorescence, the field was totally dark.
This indicates that the methanol extracted all compounds that could fluorinate with fluorochrome (NP).
Additionally, the solvents were included ethyl ether that extracts aglycons and n-butanol that extracts conjugated flavonoids. The cross sections of the leaf showed fluorescence in the adaxial epidermis presented less fluorescence and while in the abaxial epidermis it continued to present the typical fluorescence of flavonoids, which indicates that they could be conjugated flavonoids. This was corroborated by subjecting the sections to n-butanol where the fluorescence of the abaxial epidermis of the mature leaf disappeared and the adaxial epidermis showed positive fluorescence, and when both solvents were applied the yellow-orange fluorescence disappeared. These results show that free and conjugated flavonoids are found naturally within the analyzed plant material, Table two shows the flavonoid content detected by liquid chromatography.
In the ether extract of the mature leaf, 1352 mg * Kg-1 of flavonoids and 1258 mg * Kg-1 were obtained in the n-butanolic extract. Quercetin was the most abundant flavonoid followed by myricetin and kaempferol respectively.
In contrast, the immature leaf presented a lower accumulation of flavonoids in both extracts. These results confirm the hypothesis that the immature leaf presents a lower concentration of these metabolites in the anatomical sections observed compared to the mature leaf that presents fluorescence in both epidermis Feucht et al.9 and Bilyk et al.17
These results coincide with Lozoya et al.6 Seshadri and Vasista,18 authors who mention that guava shows a large amount of free and conjugated quercetin, but do not indicate in which part of the tissue they are located Markham et al.7 However, some authors mention that flavonoids are found in structures such as: epidermis as a defense strategy, glands as a detoxification reservoir and as a defense against herbivore and additionally as protectors from UV light functioning as co-pigments (Table 2).1
Abstract |
Myricetin |
Quercetin |
Luteolin |
Kaempferol |
Apigenin |
Subtotal |
Mature leaf |
||||||
Ethyl ether |
68 |
1269 |
7 |
83 |
ND |
1352 |
n-butanolic |
81 |
1083 |
ND |
67 |
27 |
1258 |
Subtotal |
149 |
2352 |
7 |
150 |
27 |
2610 |
Immature blade |
|
|
|
|
||
Ethyl ether |
45 |
665 |
5 |
69 |
ND |
784 |
n-butanolic |
267 |
894 |
16 |
55 |
ND |
1232 |
Subtotal |
312 |
1559 |
21 |
124 |
ND |
2016 |
Table 2 Flavonoid content in mature and immature guava leaf (mg * Kg-1)
ND, not detected
The results obtained in three stages: a) descriptive analysis in anatomical sections with fluorescence, b) histochemical test and c) chromatographic analysis indicate the presence of flavonoids. Said evidences show the contrast in the flavonoid content between the mature leaf with the highest concentration and the immature leaf with the lowest concentration.These results coincide with what the following authors mention Feucht et al.9 Day and Vogelmann,1 Conde et al.19 Feucht et al.9 The results obtained by liquid chromatography of the extracts of both leaves showed quercetin as the majority flavonoid followed by myricetin, luteolin, kaempferol and apigenin. These results coincide with Vargas-Alvarez et al.20 who mentions that according to the phenological stage the concentration of flavonoids changes. And according to the stress in which they are in the environment, the physiological response of the guava plant structures will be.21
Flavonoids have an important role in human nutrition, for example health promoters;22,23 Bilyk et al.17 The most important source of flavonoids in the human diet includes black tea, onions and apple and an average daily serving of 30 mg * day-1 is estimated.23 All of these sources have quercetin and kaempferol glycosides. The concentrations of these flavonoids found in the guava leaf are related to those mentioned above and these make the leaves mature due to the exposure of the variation of the environment as indicated by Benhamou and Nicole,24 Buchholz et al.14 Day and Vogelmann;1 a promising material to be used in human nutrition as a nutracentric or food additive.
The extracts of mature and inmature leaves showed quercetin as the majority flavonoid followed by myricetin, luteolin, kaempferol and apigenin The mature leaf contains more flavonoids than the immature leaf, the blue flowering, deduces other phenolic compounds not analyzed in this study
Authors want to thank to Farmers interviewed.
Authors declare no conflicto of interest exists.

©2021 Alvarez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.