eISSN: 2576-4462


Research Article Volume 5 Issue 2
1Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Campeche, México
2TECNM/Instituto Tecnológico de Chiná, México
3Instituto Tecnológico de Mérida, México
4Instituto Tecnológico Superior del Sur del Estado de Yucatán, México
Correspondence: Norma Laura Rodríguez Ávila, TECNM/ Instituto Tecnológico de Chiná, Calle 11 entre 22 y 28, Col. Centro, Chiná, Campeche, 24050, México, Tel 52(981)8272082, Ext. 108
Received: February 18, 2021 | Published: March 1, 2021
Citation: Ramírez-Benítez JE, Rodríguez-Ávila NL, Lizama-Uc G, et al. Evaluation of the potential control of natural compounds against anthracnose in mango (Mangifera indica L. cv. tommy atkins). Horticult Int J. 2020;5(2):43-49. DOI: 10.15406/hij.2021.05.00202
Anthracnose produced by Colletotrichum gloeosporioides is a post-harvest mango disease causing big economic losses worldwide. Plant pathogens have increased their resistance to chemical fungicides. The use of natural formulations for disease control represents a healthier alternative. Therefore, testing new natural antimicrobial agents is necessary.
Several natural agents for phytopathogen control have been described. Their effectiveness depends on various factors such as their composition, concentration and the environment. We evaluated the antifungal activity of organic crude extracts of Citrus x paradisi (grapefruit), Citrus reticulata (tangerine) and Citrus aurantium (sour orange) and a treatment with chitosan in mango fruits cv. Tommy Atkins against C. gloeosporioides.
The extracts obtained with hexane from dried sour orange peels had a considerable fungicidal effect on the radial growth of the fungus. Also, the parameters evaluated in mango fruits demonstrate that the chitosan treatment delays the development of symptoms of anthracnose.
Keywords: anthracnose, Colletotrichum gloeosporioides, citrus peel extracts, chitosan, mango fruit
Mexico is the main exporter of mango, contributing 20.5% to the total world in 2013.1 The search for strategies to increase the profitability of its cultivation is of increasing interest.
One of the main problems in the reduction of the sale price is the low quality of the product derived from the presence of diseases caused by contamination with microorganisms in the pre-harvest, harvest and post-harvest stages. An efficient management of the product in the postharvest period is the key factor for the successful commercialization of mango fruits, preserving its quality and modifying the speed of maturity, increasing its shelf life.
Anthracnose is the most important disease in mango production. This disease causes significant losses in mango production, and affects the quality of the fruit in the post-harvest stage of the fruit, due to the lack of phytosanitary controls during cultivation and inadequate storage management.2 Anthracnose is mainly caused by two species of phytopathogenic fungi, Colletotrichum gloeosporioides and C. acutatum, the first being the most common in most cases.3
The fungi of the genus Colletotrichum are one of the most important plant pathogens in the world, occurring mainly in tropical and subtropical regions.4 In order to solve this problem, a great diversity of products and methods for the control in field and post-harvest were designed to prevent or cure the disease, influencing the distribution of the phytopathogen, the degree of colonization in the fruits and their subsequent deterioration.
On the other hand, the main strategy for the control of fungal diseases is the use of synthetic fungicides. However, these control agents prove to be generally costly, their residues are pollutants and cause deleterious effects on other microorganisms, causing damage to both ecological and environmental levels.5–8
Similarly, the use of agrochemicals for disease control is regulated under strict national and international standards for phytosanitary control, which promotes the development and use of environmentally friendly and consumer-friendly production and storage strategies. Natural products have proven to be effective and prove to be a safe alternative for disease control.
The antimicrobial activity of citric alcoholic extracts and Citrus Essential Oils (CEO's) as well as numerous plant species has been well studied, proving that extracts of leaves, flowers, stems, roots, seeds and pericarp of the fruits have biological activity against various microorganisms in in vitro experiments.9–12
There are several studies that demonstrate that the chemical composition of alcoholic extracts and essential oils extracted by cold pressing are similar13 so the observations derived from the study of both sources of metabolites and their antimicrobial potential is of great importance. In agar diffusion and culture media (agar dilution) studies, the antifungal potential has been demonstrated for various crude vegetable extracts.13–18
Therefore, the first objective of this research was, to evaluate the effectiveness of citrus extracts grown in the State of Campeche, Mexico, in the control of anthracnose in mango fruits. Organic extracts were obtained from dried peels of Citrus x paradisi, C, reticulata and C. aurantium and evaluated using the agar dilution bioassay in the control of C. gloeosporioides.
There are different mechanisms at postharvest level to control microorganisms such as the addition of inhibitory agents (these being salts or antibiotics), heat treatments, irradiation, nitrogen sweeps, aluminum coatings, drying and vacuum storage. Protective biopolymer coatings have shown advantages of use in fruits and vegetables, since they are biodegradable and sustainable because of agroindustrial and food residues, and in some cases the coating is edible and harmless to the consumer.19
Organic coatings are especially important because they create a physical barrier to gases by producing a modified oxygen-reducing atmosphere (avoiding oxidation) and by increasing the concentration of carbon dioxide on the surface of the product.21
Several studies report the use of chitosan (deacetylated chitin) in the elaboration of edible films to conserve food. Chitosan films have been shown to be impervious to oxygen, aromas and oils, in addition to promoting the integrity, appearance, texture and brightness of the fruit.22–26,16 Chitosan has the ability to adhere to electronegatively charged surfaces, giving it unique biological, physiological and antimicrobial properties.25,27 The biological activity of this compound depends on a number of factors, including the degree of deacetylation, molecular weight, pH of the medium and temperature. Chitosan-sensitive fungi present inhibition of spore germination, morphological and ultrastructural alterations of hyphae, as well as reduction in the production of toxins. In addition, chitosan has antimicrobial capacity, due to the interaction of chitosan with the bacterial cell membrane, through amino groups in the polymer, causing alterations in proteins and other components of the cell membrane.28,29
Therefore, in order to complement this study and to have the experimental bases that allow the future application of citrus extracts assessed in the form of edible coatings on mango fruits, we evaluated the effect of chitosan on the protection of fruits of mango against the development of anthracnose symptoms under postharvest storage conditions.
Fungal strains
Strains of Colletotrichum gloeosporioides were isolated from sections of mango peel (Mangifera indica var. Tommy Atkins) with evident lesions of anthracnose. Each of the sections were seeded directly into nutrient medium Potato Dextrose Agar (PDA), incubated at 28 °C. Spores were isolated until monosporic cultures were obtained. The purified isolates were characterized morphologically making macro and microscopic description of the pathogen, including color and form of the colony, appearance and color of the mycelium; Shape, size, segmentation and sporulation of conidia.4
To maintain pathogenicity of the fungus, periodic inoculations and reisolations from infected mango fruits were carried out. Experiments were carried out using 10-15 days old cultures. For the assays, the sporangial suspension concentration was estimated using a cellcounting chamber and adjusted to 2 × 106 spores mL-1.30
Plant material
Three citrus species abundant in the Yucatan Peninsula were selected: grapefruit (Citrus x paradisi), tangerine (Citrus reticulata) and sour orange (Citrus aurantium). The fruits of these species were acquired in local markets of the State of Campeche, México For the evaluation of the coatings with chitosan, mango fruits (M. indica var. Tommy Atkins) were harvested from an orchard in the community of Castamay, Campeche, Mexico with a maturation state of 3/4 (brown-reddish color covering three quarters of the surface of the fruit).
After the collection, the peduncle of the fruits was cut. The selection of the fruits was carried out weighing the fruits collected, obtaining the population distribution of weights. Only fruits with a variation smaller than one standard deviation of the population mean were used (fruits, X = 373.5 g, σ = 56.8 g).
In all studies, the fruits selected were washed with mild detergent and sponge, rinsed in running water and disinfected with 1.5% sodium hypochlorite solution. After disinfection, the fruits were rinsed in running water, dried with an absorbent cloth and stored under refrigeration at 4 °C until use.
Preparation of plant extracts
The sour orange, tangerine and grapefruit peels were washed and disinfected as described above. This done, they were cut with scissors in pieces of approximately 1 cm2 and dehydrated at 70 °C until obtaining a constant weight. The extraction was performed with 99% ethanol, methanol or hexane, previously grinding a known quantity of the dried samples and adding the solvent using a ratio of 1:4 and mixing using an industrial mixer for 30 mins. The flask where allowed stand for 72 h and mixed occasionally.
Using a millimeter mesh, the obtained extract was filtered in order to remove the greater amount of organic matter. The alcoholic extracts were allowed to settle in a separatory flask for about 1 h, in order to obtain a clarified supernatant. The supernatant was recovered and centrifuged for 30 min at 3000 rpm to complete the clarification of the extracts. The recovery of the solvent was carried out by steam distillation using a rotary evaporator until complete removal of the solvent. The crude extracts obtained were recovered and stored in sterile amber flasks at 4 °C until their use for the growth inhibition assay.
Determination of antifungal activity
Minimal inhibitory concentration (MIC) was calculated using the agar dilution method (poisoned food technique). For this purpose, PDA medium was poisoned with different concentrations of the extracts obtained, from 250 μg / ml to 20 mg / ml. These mixtures were emptied in Petri dishes and inoculated with a strain of Colletrotrichum gloesporioides previously characterized by their high virulence and incubated at 28 °C for 20 days, verifying the absence/presence of mycelial growth and the diameter of the colonies detected both In the control as in the test assays. The results were expressed as the average diameter ± Standard Deviation (S.D.) of mycelial growth observed over time (days 2, 5, 7, 14 and 20). Each experiment was performed in triplicate and sterile water was used as the control.
Treatments of mango fruits with chitosan
Chitosan with a deacetylation degree of 75 (C12H24N2O9) from shrimp shell (SIGMA. Cat. No. C3646), diluted in 0.2 M acetic acid, pH 4, was used to obtain a concentration of 0.25% mixing by stirring at 100 °C for 60 min, to be subsequently filtered with gauze and used immediately for the treatment of the fruits.
The mango fruits were submerged in the chitosan solution or in 0.85% saline solution (negative controls) for 5 min. Subsequently, the excess liquid was removed and placed in plastic trays with cardboard beds for fruit packaging. The trays were covered with plastic film and arranged in an ambient chamber regulated at 21 °C, relative humidity of 40% and darkness, for 10 and 30 days. Other mango fruits were sprayed with approximately 15 mL of C. gloeosporioides spore solution (1x106 spores/mL) or saline solution (positive control to infection). Finally, to evaluate the antagonistic effect of chitosan on C. gloeosporioides infection, mango fruits treated 24 h prior to chitosan were sprayed with spore suspension of C. gloeosporioides and incubated in an environmental chamber. All treatments were performed in quadruplicate (n = 4 mangos per replicate). Photographs were taken of the mangoes after incubation to determine the disease progression.
After treatment, the mangoes were dissected to separate two portions of pulp from the seed. With a bronze punch, 6 cylinders of the fruit were obtained with 1 cm in diameter, distributed randomly along the pulp sections. Subsequently, the exocarp (cuticle or peel) and mesocarp (pulp) were separated and collected separately, taking 13 mm of the cylinder adjacent to the peel. Samples from each of the mangos represented individual samples.
The exocarp (peel) and mesocarp (pulp) of the sampled mangoes were rapidly frozen by immersion in liquid nitrogen and ground in mortar until a pulverized sample was obtained. The ground samples were stored separately in plastic bottles at a temperature of -40 °C for further evaluation.
Determination of total phenolic contents
200 mg of pulverized pulp or peel was taken and 500 μl of a mixture of ethanol: methanol (1:1, v/v) was added, mixed vigorously and incubated with stirring for 30 min at 25 °C. The samples were then centrifuged for 10 min at 13,000 rpm, collecting the supernatant. The extraction process was repeated with the sediment, collecting the supernatants of each cycle (3 cycles in total). The organic extracts obtained were dried in a rotary evaporator under vacuum at an absolute pressure of 400 mm Hg and 45 °C for 20 min. The dried samples were resuspended in 500 μL of deionized and degasified water. The determination of phenolic compounds was carried out by the Folin-Ciocalteu method. 29
Then 20 μl of the sample was mixed in 1.58 mL of water and 100 μL of the Folin-Ciocalteu reagent, and vigorously mixed for 10 min. 300 μl of 20% sodium carbonate was added, mixed and incubated for 2 hours at 25 °C. The absorbance at a wavelength λ = 765 nm was measured. The results were reported in g of phenols/kg expressed as fresh weight using a calibration curve generated using pyrocatechol.
Determination of the moisture content of the fruit, titratable acidity and pH
The treated mangoes were weighed during the experiment (0, 10 and 30 days of treatment) and the weight difference was calculated according to the following equation:
The determination of the acidity was carried out by means of titration by sodium hydroxide (NaOH) 0.1N (Diario Oficial de la Federación, 1978), using 0.75% phenolphthalein as an indicator. 5 g of fresh pulp was homogenized with 50 mL of deionized water for 60 s. The mixture was filtered through 4 layers of gauze and the filtrate was collected. 10 mL of the filtered juice were titrated and the data were reported as mL of NaOH 0.1 N consumed/100 g of pulp. The pH was determined with a portable potentiometer.
Determination of the profiles of reducing sugars in pulp
100 mg of pulverized pulp was taken and 1000 μL of distilled water was added. The mixture was vigorously shaken and incubated at 50 °C for 15 min. The suspension was centrifuged at 13,000 rpm for 15 min and the supernatant was recovered. The pellet was resuspended with 1000 μL of distilled water, repeating the process. The tubes were centrifuged again at 13,000 rpm for 15 min. The two supernatants of each sample were mixed. Reducing sugars were determined by the colorimetric method of Dinitrosalicylic Acid.32 300 μL of 1% DNS solution and 300 μL of the sample were added. The tubes were heated to 90 °C for 15 min, the samples were allowed to cool to room temperature and 100 μL of 40% sodium tartrate solution was added. The absorbance of the samples was measured at a wavelength of λ = 575 Nm, using a visible light spectrophotometer. The reducing sugar concentration of each sample was calculated using the linear regression adjustment obtained from the glucose standards, reporting them as glucose/kg fresh weight.
Determination of sugar/acid ratio
To determine the degree of maturity of the fruits submitted to the different treatments, the ratio between the concentration of reducing sugars and the titratable acidity was calculated according to the following equation:
The ratio in equivalent grams of glucose was reported on equivalent grams of citric acid. For the conversion of units to the titratable acidity, the conversion factor was used 1 mL of 0.1 N NaOH consumed equals 0.006404 g of anhydrous citric acid.33
The quantitative values of the physicochemical and biochemical tests of the chitosan bioassays were represented as means ± Standard Deviation of the replicates of each treatment. Statistical differences were determined using an ANOVA test using the Holm-Sidak algorithm, with a significance level of P <0.005. Statistical analysis was performed using SigmaStat v. 3.1 (Systat Software Inc., San Jose, CA, USA).
As is shown in Figure 1A, crude extracts obtained with hexane were generally more effective in inhibiting the growth of mycelium of C. gloeosporioides (Figure 1B). Among peel extracts, C. aurantium had the most effective antifungal effect, with concentrations as low as 250 mg/ml inhibiting at least 3 times the growth of mycelial diameter than observed in the bioassays with the crude extracts of grapefruit and tangerine (Figure 1B). This represents 75% inhibition of growth, much higher than that reported by Hernández-Albíter (19) who report a 40% reduction in germination of conidia of this phytopathogen when applying crude extracts of Citrus aurantium at different concentrations to conidial dilutions and Madhuri y cols. (20), who proved that the methanolic extracts of sour orange inhibited mycelial growth to>50% of C. capsici.
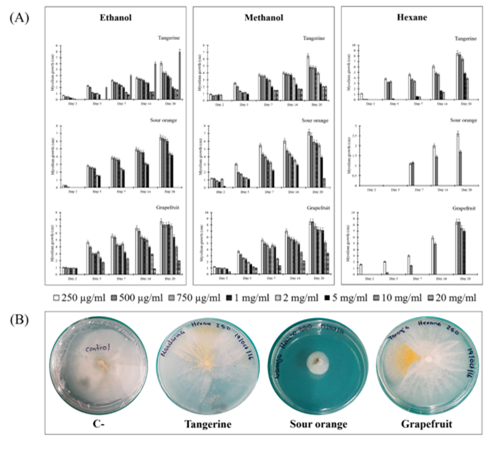
Figure 1 (A) Effect of organic extracts obtained with ethanol, methanol and hexane from dried peels of tangerine, sour orange and grapefruit on the growth of mycelium of Colletotrichum gloesporioides.(B) Effect of different concentrations after 20 days of the crude extract obtained with hexane from sour orange dried peels on linear growth (cm) of the mycelium of C. gloeosporioides. C-: Negative control (sterile water).
With respect to the treatment with chitosan, it was observed that all the fruits presented have a similar appearance to the 10 d post incubation (Figure 2A). However, at 30 days and specially in the fruits exposed only to the solution of spores of C. gloeosporioides, it is possible to note the presence of lesions characteristic of anthracnose, such as the appearance of spots on the epidermis and the darkening of the layer surface of the pulp. On the contrary, in fruits treated with chitosan prior to inoculation with the phytopathogen, these lesions are not observed so markedly (Figure 2A).
The analysis of the total phenol concentration showed a greater amount of these compounds in the pulp of the mangos treated with chitosan (Figure 2B) in contrast to the treatment with the phytopathogen only, where no appreciable increase of these compounds was observed. The effect detected in chitosan treatment was noticed even in the treatment with chitosan and the phytopathogen (Figure 2B, treatment 5), suggesting that chitosan causes some change in fruit metabolism, inducing the production of these compounds.
As can be seen in Figure 2C, the concentration of total phenols is much higher in the skin compared to the pulp. It was observed that the amount of total phenols in the skin decreased drastically in treatments that included exposure to chitosan, with the lowest phenol levels at 30 d of incubation (Figure 2C, treatment 2). This effect was not observed in fruit skin inoculated only with C. gloeosporioides, where no changes were induced in total phenol content (Figure 2C, treatment 4). However, in mangoes treated with chitosan and subsequently with phytopathogen present a dramatic decrease in total phenol content (Figure 2C, treatment 5).
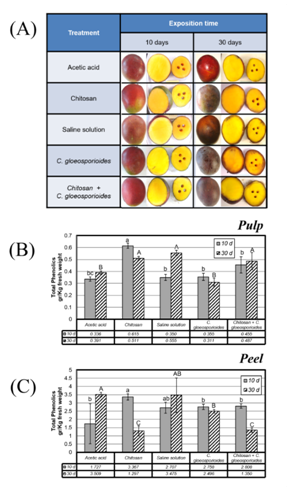
Figure 2 A) Physical appearance of fruits after different treatments for 10 and 30 days (d). B) Concentrations of total soluble phenols in mango fruit pulp subjected to different treatments and incubated for 10 d (gray bar) and 30 d (shaded bars). C) Concentrations of total soluble phenols in mango fruit peel submitted to different surface treatments and incubated for 10 d (gray bar) and 30 d (shaded bars). Bars with different letters means significant differences, resulting from one-way ANOVA (P <0.005).
Regarding the loss of moisture, during the first 10 days of treatment no statistically significant differences were observed in this variable, losing on average between 2.00 and 2.55% of humidity with these treatments (Figure 3A). During the second sampling (30 days) it was observed that the mangos treated with a chitosan coating showed a loss of moisture lower than those to which it was not applied. Specifically, fruits treated with chitosan exhibited a loss of moisture of 5.22% (Fig. 3A, treatment 2) while their negative control (acetic acid solution without chitosan) had a moisture loss of 8.62% (Figure 3A, treatment 1).
The protective effect of chitosan coating on mango fruits was consistent with that expected from an organic coating23 preventing moisture loss and weight in the post-harvest stage. The fruits inoculated with C. gloeosporioides showed the highest moisture loss, 9.84% (Figure 3A, treatment 4), an event associated with the induction of fruit maturation due to the presence of the phytopathogen (3). In turn, it was observed that chitosan-treated mango fruits inoculated with the phytopathogen had the lowest moisture loss, suggesting that the coating with chitosan had a protective effect on the fruit against infection with C. gloeosporioides.
It should be noted that the protective effect of chitosan was reached with a much lower concentration (0.25%) and at a higher temperature (21 °C) than that commonly used in existing reports (15 °C), where the protective effect on mango fruit quality (cv. Tainong) was demonstrated at 2%35 or only through in vitro bioassays.36
When analyzing the titratable acidity present in the pulp of treated mango fruits, it was observed that infection with C. gloeosporioides produces alkalinization of the pulp at 30 d of treatment (Figure 3B, treatment 4). Over time, this alkalinization was presented to a lesser extent in the treatments in which chitosan was applied, which suggests a decrease in the ripening rate when the fruits are treated with this compound, especially if the previous treatment is performed previous the infection with C. gloeosporioides (Figure 3B, treatment 5). When measuring the pH of mango pulp, it was observed that only the treatment with C. gloeosporioides causes alkalinization of the tissue (from 4.06 on day 10 to 5.0 on day 30, data not shown).
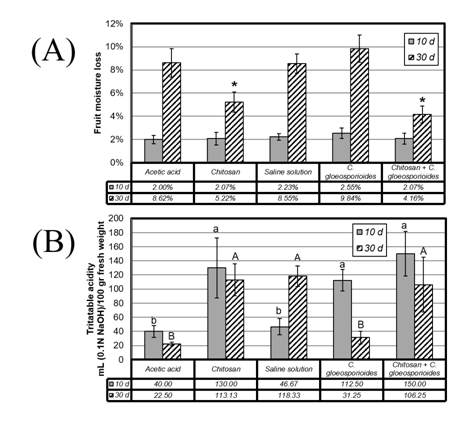
Figure 3 A) Moisture loss in mango fruits subjected to different treatments and incubated for 10 d (gray bar) and 30 d (shaded bars). Asterisks indicate significant differences, resulting from one-way ANOVA (P <0.005). B) Titratable acidity contents of mango pulp on fruits submitted to the different treatments and incubated for 10 d (gray bar) and 30 d (shaded bars).Bars with different letters means significant differences, resulting from One-Way ANOVA (P <0.005).
This data together with the effect observed in the determination of titratable acidity of mango fruits at 30 d of treatment, indicates that both the acidity due to the presence of organic acids and that due to the release of hydrogen ions were affected by the infection of the plant pathogen. Organic acids (tartaric, citric, succinic), essential components in the tricarboxylic acid cycle, are metabolized during fruit respiration.35 A decrease in acidity has been observed during ripening of many fruits, suggesting the existence of considerable metabolic rates at this stage.36 At the same time, organic acids and their derivatives contribute greatly to the taste, with a strong correlation between the sugar: acid balance and the sensorial perception of the consumer in the different species of fruit trees.37 It is noteworthy that the treatments that included the coating with chitosan maintained the acidity of the pulp, whereas the positive control of infection with C. gloeosporioides decreased the acidity of the fruit, suggesting an increase in the consumption of organic acids as a consequence of the invasion of the fungus in the pulp.
The sweetness of the mangoes is an important parameter of their quality and is correlated with the degree of ripening of the fruits and the sugar/acid ratio. Evaluating this factor in the pulp of the fruits submitted to the different treatments, it was observed that during the first 10 d the treatment with chitosan induced a slight increase in the concentration of reducing sugars, compared to the other treatments (Figure 4A, treatments 2 and 5). However, at longer incubation time, it was observed that this condition was reversed, with chitosan-treated mangoes having the lowest concentration of reducing sugars in the pulp (Figure 4A, treatments 2 and 5). Negative controls (acetic acid) showed a gradual increase in the concentration of reducing sugars, which is to be expected in the normal fruit ripening process. Those mangoes that were infected with C. gloeosporioides showed a dramatic increase in the concentration of reducing sugars at 30 d, suggesting the induction of fruit ripening resulting from the development of the disease. However, it is important to note that in the fruits that were coated with chitosan prior to the inoculation with the phytopathogen, a significant increase in the reducing sugars was not observed throughout the experiment, suggesting a retarding effect of the fruit maturation and the development of anthracnose (Figure 4A, treatment 5).
As can be seen in Figure 4B, the sugar/acid ratio increases during the experiment in the negative controls (acetic acid and saline). This provide experimental evidence about the development of fruit maturation, which involves the consumption of organic acids increasing the respiration rate as well as a cleavage of reserve polysaccharides and the production of simple sugars.
In fact, the values of the sugar/acidity ratio in the chitosan-coated fruits are statistically lower than those without chitosan, maintaining a constant maturity rate up to 30 d of treatment, even in fruits inoculated with phytopathogen spores (Figure 4B, treatment 5).
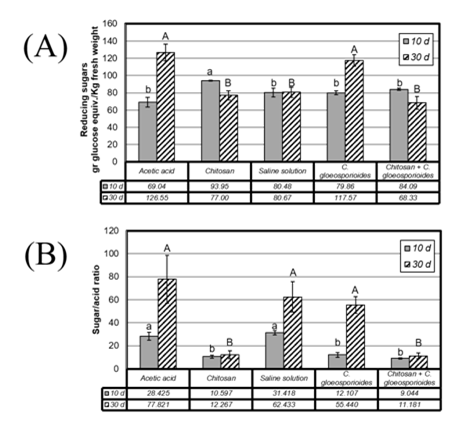
Figure 4 A) Concentrations of reducing sugars in the pulp in mango fruits submitted to different treatments and incubated for 10 d (gray bar) and 30 d (shaded bars). B) Sugar/acid ratio in the pulp in mango fruits submitted to the different treatments and incubated for 10 d (gray bar) and 30 d (shaded bars). Bars with different letters means significant differences, resulting from One-Way ANOVA (P <0.005).
It is also possible to observe that infection with phytopathogen causes a greater increase in sugar/acidity ratio, suggesting an accelerated cleavage of reserve polysaccharides by the action of the hydrolytic enzymes of the invasive fungus.37
It has been shown that the coating with chitosan forms an effective barrier against the loss of moisture and volatile organic compounds in strawberries and other fruits, resulting in an increase in shelf life.23,26,27,34 This suggests that this barrier could interfere with the natural metabolism of the fruit altering the respiration rate and the production of ethylene resulting in a delay in maturation.
In conclusión, the citrus peels are considered waste products. Our results indicate that the peels of the Citrus species bioassayed present antimicrobial properties. Specifically, the peel extracts of C. aurantium obtained with hexane showed the higher bioactivity against C. gloeosporioides from a dose of 250 g/ml. Therefore, it is possible to postulate that the peels of this citrus fruit can be used to obtain extracts that could be applicable for field and post-harvest control of anthracnose. However, it is necessary to carry out new field/in vivo bioassays to verify its usefulness in the control of this disease.
Additionally, the results show that the coating with chitosan applied to mango fruits has a protective effect to the infection with C. gloeosporioides, increasing the shelf life of the fruit under storage conditions. It should be noted that the mango is a climacteric fruit, which implies that the maturation process is triggered by a sudden increase in the rate of respiration and ethylene production, so post-harvest management strategies aimed at the prolongation of shelf life are of special interest for the agroindustrial chain of this fruit, which usually involves long periods of storage in cold with the objective of delaying the maturation of the fruit increasing the costs of production and diminishing the profit margin of the intermediary. This is the first work that reports the increase in shelf life of this product, in environmental condition exposed to the phytopathogen causing anthracnose, by organic methods free of agrochemicals.
This work was supported by the Tecnológico Nacional de México [Grant number 254.15-PD].
Authors declare no conflict to of interest exists.

©2021 Ramírez-Benítez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.