eISSN: 2576-4462


Clinical Paper Volume 2 Issue 2
1ICAR- Central Institute of Post-Harvest Engineering and Technology, India
2Department of Biochemistry, Chaudhary Charan Singh Haryana Agriculture University, India
Correspondence: Poonam Choudhary, ICAR- Central Institute of Post-Harvest Engineering and Technology, Punjab-141004, India
Received: November 25, 2017 | Published: April 17, 2018
Citation: Choudhary P, Jain V. Effect of post-harvest treatments of selenium on physico-chemical quality in guava (Psidium guajava L.) fruit. Horticult Int J. 2018;2(2):41-44 DOI: 10.15406/hij.2018.02.00024
Guava fruits of cv. Hisar Surkha (shelf life 3-5 days) were harvested at mature green stage. The fruits were given post-harvest treatments of selenium (0.01 ppm, 0.02 ppm, 0.03 ppm, 0.04 ppm and 0.05 ppm) each for five minutes. The fruits were analyzed for physcio-chemical quality parameters viz. physiological loss in weight, firmness, total soluble solids and titratable acidity at three days interval until complete decay. The data revealed that firmness and titratable acidity decreased continuously while the physiological loss in weight increased progressively during storage. Total soluble solids increased progressively with increasing storage period upto 6 DOS stage thereafter, it declined at 9 and 12 DOS stages respectively in control fruits. Pretreatment of guava fruits with 0.02 ppm of selenium resulted in significantly delayed decline in physiological loss in weight (30.44%), titratable acidity (7.90%) and retained the fruit firmness (34.13%) as compared to control fruits. The total soluble solids were also maintained in guava fruits treated with 0.02 ppm selenium at all stages during storage. The delay in reduction of firmness, titratable acidity and weight loss by lower selenium concentrations positively affects fruit storage.
Keywords: firmness, guava, physiological loss in weight, selenium, total soluble solids, titratable acidity
Guava (Psidium guajava L.) is one of the important commercial fruits that belong to family Myrtaceae. It is native to tropical America and presently found in many tropical and subtropical countries. In India, it ranks 5th in cultivated area after mango, banana, citrus and apple. Guava is very rich in minerals viz. iron, phosphorus and calcium and is a good source of ascorbic acid, dietary fiber, carotenoids, phenolic compounds, sugars, pectin and lipids.1–4 It is a climacteric fruit5 which ripens rapidly after harvest, and loses its texture and quality in 3-4 days at room temperature. It contains a high percentage of their fresh weight as water and consequently exhibit relatively high metabolic activity which continues post-harvest and makes it highly perishable commodity.6 Its soft skin makes it susceptible to bruising and mechanical injury due to which it cannot be stored for more than a week even during winter season. The susceptibility of fresh produce to post-harvest diseases and deterioration of quality attributes increase after harvest and during prolonged storage.7 To avoid a glut and the reduce per cent losses in guava, it becomes desirable to evolve technologies for prolonging its keeping quality through delaying the softening process so as to improve the opportunity of its transportation to distant markets. Therefore, efficient measures are desirable to increase shelf-life to facilitate long distance transportation, increase marketable period and thereby to improve commercialization of the fruits. Chemicals such as polyamines,8 gibberelic acid,9 CaCl2,10 carboxy methyl cellulose11 and ascorbic acid12 have been reported to improve the shelf-life and quality of fruits. Selenium is an important element associated with the antioxidant activity.13 It is effective in delaying plant senescence and some antioxidative losses due to enhanced activity of glutathione peroxidase.14 Pear fruits treated with selenium maintained fruit firmness, total soluble solids, sugar to acid.15 It has been shown to be effective in decreasing the production of ethylene, consequently improving the quality and the shelf-life in lettuce and chicory16 and tomato.17 It has a positive effect on plant protection against abiotic stress in plants at low concentrations where it acts as an antioxidant.18 Keeping these facts in view, we report here changes in physico-chemical quality parameters in guava fruits treated with selenium during storage.
Plant material, treatments and environmental conditions
Guava fruits cv. Hisar Surkha were harvested at mature green stage from the Horticulture Farm, CCS Haryana Agricultural University, Hisar. The fruits of uniform size and color were selected and dipped in aqueous solution of sodium selenate (0.01 ppm, 0.02 ppm, 0.03 ppm, 0.04 ppm and 0.05 ppm for 5 min. The control fruits were dipped in water for the same duration. The fruits were then air dried and stored in cardboard boxes at room temperature and analyzed at three days interval until complete decay. All the chemicals and biochemicals used in the present study were of analytical grade and procured from E. Merck (Bombay), Himedia Laboratories Ltd. (Bombay), Sigma Chemical Company (USA), and Sisco Research Laboratories Pvt. Ltd. (Bombay). Physiological loss in weight (%): Weight of freshly harvested fruits was recorded at 0 day of storage and termed as initial weight. On each day of observation, the stored fruit were again weighed and termed as final weight on that particular day of observation. The physiological loss in weight (PLW) on each sampling date was calculated using the following formula:

Fruit firmness
Fruit firmness was measured by hand held fruit pressure tester penetrometer tester (Model FT 327; TR Agricoli, Italy), using cylindrical plunger of 8 mm diameter and firmness scale of 13 kg/cm2. The firmness was measured from each side of the equatorial region of the fruit. Firmness of five fruits per treatment was measured and expressed in kg/cm2.
Total soluble solids (TSS): Total soluble solids were determined by using Abbe’s hand refractometer of 0-32 (°Brix) range at room temperature and expressed as (°Brix) soluble solids of fruits.
Titratable acidity: Total acids were estimated by titration against 0.1 N NaOH.19 Five grams of fruit pulp was macerated in 5 ml of distilled water. Its volume was made to 100 ml with distilled water. It was shaken thoroughly and filtered through Whatman No. 4 filter paper. An aliquot of 20 ml was titrated against 0.1 N NaOH using 1% phenolphthalein as an indicator. Appearance of pink colour was observed. From the volume of alkali used, acidity was calculated and expressed as g citric acid /100 g fruit pulp.
![]()
<p>Eq. wt. of citric acid = 64.04</p>
<p>The data obtained in the present investigation were subjected to statistical analysis of variance (ANOVA) technique using completely randomized designs (CRD). The critical difference at 5% levels of significance was calculated and used for making comparison among different treatment during storage. All experiments were replicated three times and ‘Statistical Package for Agriculture Scientists’, OPSTAT software, CCS Haryana Agricultural University.</p>
Loss in weight in fresh fruit and vegetable is mainly due to the loss of water caused by transpiration and respiration processes.20 It is a major deciding factor of visual quality loss of agricultural products. In our study, Results presented in Figure 1 demonstrate that physiological loss in weight (PLW) increased progressively and significantly from 3.28 to 16.02% as the period of storage increased from 3 to 12 days in guava fruits. Loss in weight was significantly lesser in fruits treated with lower concentrations (up to 0.03 ppm) of Se. The application of 0.01 ppm and 0.02 ppm Se was more effective in delaying the PLW resulting in 21.30% and 30.44% decline in PLW respectively as compared to control fruits during storage. The PLW exceeded the 10% threshold in control (12.86%) and Se (0.03 to 0.05 ppm) treated fruits (11.33 to 14.30%) at 9 days of storage (DOS), whereas the fruit treated with 0.01 ppm (9.95%) and 0.02 ppm se (8.52%) at 9 DOS. The increase in PLW during storage has also been reported in guava5,21 and aonla.22 The PLW during storage was mainly due to evaporation of water and loss of metabolites during respiration.23 The untreated and those treated with higher Se concentration exhibited higher PLW as compared to the treated fruits during storage. This may be due to rapid shrinkage and wilting caused by water loss and due to higher rate of respiration and ethylene production at higher Se concentrations. Pezzarossa et al.17 reported that owing to its antioxidative role, Se is found in effective at low concentration in delaying the onset of plant senescence and fruit ripening through a decrease in ethylene synthesis.
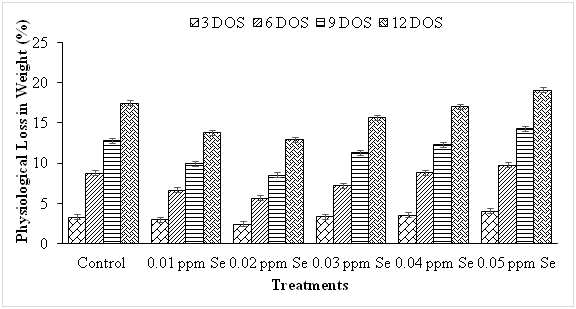
Figure 1 Effect of selenium on physiological loss in weight during storage in guava fruit. The bar (I) denotes ±SE. [CD (P≤ 0.05) 0.367 (days of storage), 0.450 (treatments), 0.899 (interactions)].
Firmness is an important attribute demanded by consumers and also used to assess the quality of fruits during maturity. In present investigations firmness of guava fruit decreased significantly and progressively with the increasing storage period from 11.40 Kg /cm2 at 0 DOS to 1.40 Kg /cm2 at 12 DOS in control fruits (Figure 2). The higher concentrations (0.04 and 0.05 ppm) of Se had negative impact on firmness thereby reducing the firmness to 0.73 and 0.40 Kg /cm2 at 12 DOS and hence resulting in loss of texture. Contrarily, lower concentrations (0.01 and 0.02 ppm) of Se could delay the softening of fruits by retaining their firmness to 2.23 Kg /cm2 and 3.70 Kg /cm2 at 12 DOS stage. These results are in accordance with the results of Guo et al.24 in nectarine, Liu et al.15 in pear and Pezzarossa et al.25 peach fruits during storage. Due to antioxidative property, Se delayed senescence thereby delaying the reduction in fruit firmness during storage.25 Guo-liang and Jian-bao26 also found a positive effect of Se on firmness in peach fruits. The total soluble solids which generally contain sugar, mineral and acids, is a reliable index to judge the proper stage of maturity. There was a progressive increase in TSS content of guava fruit with increasing storage period (Figure 3) from 11.37 at 0 DOS to 14.50°Brix at 6 DOS stage thereafter, it declined to 12.63°Brix and 11.66°Brix at 9 and 12 DOS stages respectively in control fruits. These results are in agreement with those reported earlier in guava,21 ber27 and sapota.28 Increase in TSS during storage may be due to hydrolysis of starch29 or due to the breakdown of complex polymers into simple substances by hydrolytic enzymes which might be further metabolized during respiration and thus the level got decreased during subsequent storage.30All the treatments (except 0.04 and 0.05 ppm Se) delayed the production of TSS thus resulting in lesser increase in TSS content upto 6 DOS stage as compared to that in the control. As it is evident from the data, after 6 DOS stage, there was a decline in TSS content but the reduction was significantly less and slower in the treated fruits as compared to control, 0.04 and 0.05 ppm Se treated fruits.
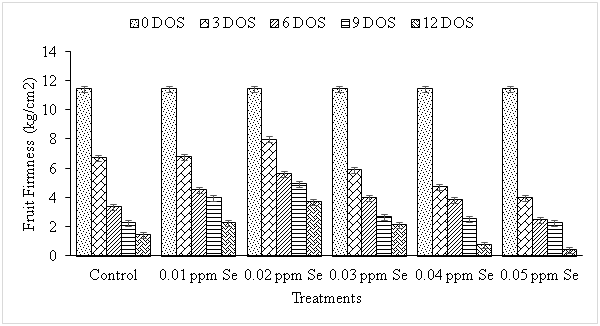
Figure 2 Effect of selenium on firmness during storage in guava fruit. The bar (I) denotes ±SE. [CD (P≤ 0.05) 0.242 (days of storage), 0.265 (treatments), 0.592 (interactions)].
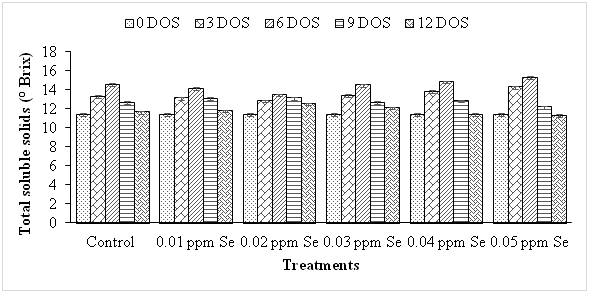
Figure 3 Effect of selenium on total soluble solids during storage in guava fruit. The bar (I) denotes ±SE. [CD (P≤ 0.05) 0.160 (days of storage), N/A (treatments), 0.392 (interactions)].
Among the treatments, 0.02 ppm Se was most effective in maintaining the TSS content from 11.37 °Brix at 0 DOS to 13.43 °Brix at the 6 DOS stage thereafter, TSS declined to 12.47 °Brix at 12 DOS stage. The declines in increase in TSS content by selenium treatment are supported by Liu et al.15 in pear fruits and Wu and Ning31 in Chinese jujube during storage. Contrary to this, addition of selenium did not affect the soluble solid content and titratable acidity in tomato fruit grown under hydroponic conditions.32,33 The titratable acidity estimates the organic acid content of fleshy fruits. Critical perusal of the data indicates that titratable acidity decreased linearly with the increasing period of storage from 0.527% at 0 DOS stage to 0.303% at 12 DOS stage in control fruits with a mean value of 0.405% (Figure 4). This might be due to conversion of acids into salts and sugars and their further utilization in respiration and metabolic processes (Ibrahim et al. 2014). The fruits treated with lower Se concentrations (0.01-0.03 ppm) had higher titratable acidity at all the stages of storage as compared to the control. The treatments with 0.04 and 0.05 ppm Se resulted in further reduction in fruit acidity to 0.388 and 0.369% and 0.282 and 0.250% respectively at 6 DOS and 12 DOS stages. Maximum titratable acidity (0.434%) was found in fruits treated with 0.02 ppm Se. The mean value of acidity increased from 0.405% in control to 0.414% and 0.437% in the fruits treated with Se (0.01 ppm and 0.02 ppm) during storage. The reductions in titratable acidity due to selenium treatment are supported by Wu & Ning31 in Chinese jujube and Liu et al.17 in pear and Guo-liang & Jian-bao26 in peach fruits during storage. However, Nancy & Arulselvi32 and Pezzarossa et al.33 reported that post-harvest Se treatment did not affect titratable acidity in tomato fruits during storage.
The present results show that progressive increase in PLW and continuous decrease in fruit firmness and titratable acidity during storage of guava fruits resulted in loss in texture and decline in fruit quality. The fruits treated with lower concentration of selenium delayed decline in PLW, TSS and titratable acidity and retained the fruit firmness during storage. Pretreatment of fruits with 0.02 ppm se could be effective in delaying the softening by maintaining the tissue structure and physico-chemical characteristics of guava fruits during storage. At lower concentrations selenium acts as antioxidant and delays the softening of fruits while at higher concentrations, it acts as pro-oxidant and showed negative effects on fruit quality parameters.
The authors gratefully acknowledge the financial support by Department of Science and Technology– INSPIRE Fellowship and Dean, College of Basic sciences, CCS Haryana Agricultural University.
We Authors hereby declare no conflicts of interest.

©2018 Choudhary, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.