eISSN: 2576-4462


Short Communication Volume 8 Issue 3
1Department of chemistry, Govt.Islamia Post Graduate College Cooper Road Lahore, Pakistan
2Beaconhouse School System, Canal Side Campus Lahore
3Beaconhouse College Program, Pakistan
Correspondence: Fauzia Anjum Chattha, Department of chemistry, Govt. Islamia Post Graduate College Cooper Road Lahore, Pakistan, Tel 092-3008578896
Received: September 20, 2024 | Published: October 4, 2024
Citation: Chattha FA, Naeem F, Ahmad S. Effect of 3-Arylindazol -1-acetic acids on plants growth. Horticult Int J. 2024;8(3):77-82. DOI: 10.15406/hij.2024.08.00307
Background: Indazole are herbicides and are used widely but their acetic acids function s still detectable that might have some prominent effects on seed germination and growth of plants. A series of 3-arylindazole-1-acetic acids was done by alkylation of 3- aryl-1H-indazoles.
Study Overview: These compounds were subjected to evaluate their biological action on wheat (Triticum aestivum) and sorghum (Sorghum bicolor) seeds germination along with early growth of seedlings.
Key Findings: The results showed that these compounds are found to be depressant of seed germination hence can be successfully used for crop seeds storage in extremely hot weather of Pakistan.
In wheat effect of these compounds on early growth is acceleration in all concentrations. In sorghum at high concentrations these compounds were proved to have inhibitory effect more pronounced on root and shoot growth while at low concentration effect is negligible in comparison to control.
Conclusion: It is concluded that 3-Arylindazole-1-acetic acids are supposed to delay the germination of seeds simply by lowering the metabolic process during the imbibitions but early growth of seed show somewhat acceleration
Keywords: germination inhibition, wheat, sorghum, abscisic acid, indole acetics acids
Indazoles and 1H-indazole are herbicides and its effect is probably growth inhibitor1-3 when applied in soil before or after seed germination and can be helpful to remove undesired plants. These compounds and their formulations are generally employed for controlling harmful plants, especially weeds in crops.4 Nitroindazoles are used as fungicides, herbicides, plant growth regulators and tetrahydro-2H-indazoles also exert a prominent herbicidal activity in application to soil before or after germination.5 Isoindoles are also quite effective plant growth regulators6 and 3-aryl-1H-indazoles are also growth inhibitor of early plant growth and can be helpful in seed storage.7
Indazole-3-acetic acids play an important role in thinning of young fruits and blossoms when applied to fruit trees. A study on different indazole-3-acetic acids and their derivatives have shown that 5-chloro-1H-indazole-3-acetic acid.
The main objective of this project is to synthesize such compounds that show maximum effectiveness from minimum dosage. This high efficiency is desirable from two-stand point’s first economy and second distribution with in the plant. Such reforms should be implemented to have more production of crops, vegetables and fruits to fulfill the requirements of huge population burden. This is only possible by synthesis of growth regulators (herbicides and insecticide) inside the country as it would be more effective in Pakistani environment and in the range of farmers. Country should be free of import of such chemicals and it will save a lot of foreign exchange.
The indazole-1-acetic acids are important plant growth regulators and we synthesized them starting from indazoles. Main purpose to synthesize the heterocyclic compounds of versatile biological active and can be useful for plant growth regulation activity, because Pakistan is an agriculture country. Our economy mainly depends upon agriculture. In fact horticulture industry is indirectly dependent on plant growth regulators as these are exclusively used for different purposes like treatment of seeds for enhancing their germination, rooting, flowering fruiting, increased yield, ripening and as a post-harvest treatments for better shelf-life. The main purpose of plant growth regulators is to increase the yield but yield does not mean increase of useless bulk so it should be known properly before application.
IR spectra were recorded on a Perkin-Elmer 1600 FT-IR spectrometer using KBr discs and Nujol mull was used in some cases. AVANCE 300 Nuclear Magnetic Resonance Spectrophotometer, Bruker Esquire 3000 + ion trap with ESI ionization and FAB+-MS spectra were used for compounds analysis. Perkin Elmer 2400-CHN analyzer and Leco CHNS analyzer CHNS-932 leco corp USA were used microanalysis
Synthesis of indazoles
For synthesis of indazoles method of Servi and Akgun was used.8 The indazole acetic acids were prepared from the corresponding indazoles using the method of Teixeira et al.9 The resulting solid was purified by recrystallization from ethyl acetate and characterized.
Seed germination inhibition studies
Effects of the synthesized compounds on the seed germination were determined using wheat and sorghum seeds of high viability. Wheat (10) and sorghum (20) seeds were placed per Petri dish soaked with distilled water (8mL) and test compounds (1-100 ppm) were sprayed on double layered filter paper. Petri dishes were placed at 25 0C in an incubator.10 The number of seeds germinated after 1, 2 and 3 days was monitored. Protrusion of radical was an index of completion of seed germination. Each experiment was performed three times. Indole butyric acid, abscisic acids and control (without any chemical) were used as standards. The number of secondary roots in wheat seedlings, root length and shoot lengths were measured by binary response variable on 3rd day and 5th day respectively. ANOVA test was used to compile the data.
A series of 3-aryl-indazole-1-acetic acids (1-9) was synthesized by N-alkylation of 3- aryl-indazoles with ethyl bromoacetate followed by base hydrolysis and then acidification with hydrochloric acid formed acids.11
The structures of 3-aryl-1H-indazoles acetic acids (1-9) were confirmed by IR, HNMR, Peaks in the region 3310 cm-1 are indicative of OH in compounds 2. While 692 cm-1 peak is N-H stretching in compound 4 and 1517 cm-1 signal correspond the N-O stretching confirms the nitro in compound 9. Peak at 692 cm-1 is carbon-alkyl stretching in compounds 5 and 6.
Effects of indazole-1-acetic acids on seed growth
Indazole-1-acetic acid and its different derivatives (Figure 1) were synthesized and tested for their seed germination and plant growth regulating activity. Data shows that various substitutions in indole-1-acetic acids have variable effects on seed germination and early growth of plants.
Effects on seed germination
Wheat and sorghum seeds were tested for the effects on seed germination and growth. In wheat (Figure 2), at 100 ppm 3-styrylindazole-1-acetic acid (3) and 3-(4ʹ-chlorophenyl)- indazole-1-acetic acid (4) showed inhibition in germination <50 % whilst others showed moderate growth at this high concentration. At 10 ppm, 5 had 50% germination. At 1 ppm 3-phenylindazole-1-acetic acid (1) 3-styrylindazole-1-acetic acid, 3-(4ʹ- acetaminophenyl) indazole-1-acetic acid (4) showed 50% and 3-(4ʹ- chlorophenyl)indazole-1-acetic acid (5) showed 60% germination (Figure 2). Effects on seed germination were more pronounced in sorghum and less in wheat.
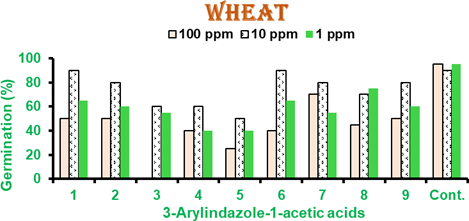
Figure 2 Effects of indazole-1-acetic acid on wheat seed germination at 25oC.
Results are mean of 3 independent experiments with 10 wheat seeds per experiment.
Error is within 5% limit. Cont. is control.
Data showed that at 100 ppm in sorghum (Figure 3) indazole-1-acetic acid, 3- styrylindazole-1-acetic acid (3), 3-(4ʹ-acetaminophenyl)-indazole-1-acetic acid, 3-(4ʹ- chlorophenyl)-indazole-1-acetic acid (5), 3-(3ʹ,4ʹ-dioxomethylenephenyl)-indazole-1- acetic acid (8) and 3-(2ʹ-nitrophenyl)indazole-1-acetic acid (9) had germination inhibiting effect and showed <50 % germination. At 10 ppm 3-phenylindazole-1-acetic acid (1), 3- (4ʹ-chlorophenyl)-indazole-1-acetic acid (5) and 3-(3ʹ,4ʹ-dioxomethylenephenyl)- indazole-1-acetic acid (8) showed inhibiting effect and others have moderate effect on germination. At 1 ppm 3-(4ʹ-acetaminophenyl)-indazole-1-acetic acid (4) and 3-(4ʹ- chlorophenyl)-indazole-1-acetic acid (5) showed germination inhibiting effect all other compounds showed 60-90% germination (Figure 3).
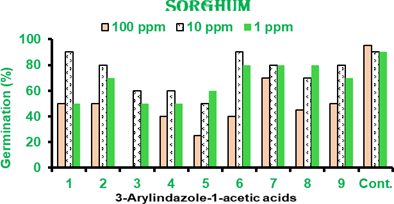
Figure 3 Effects of indazole-1-acetic acid on sorghum seed germination at 25oC.
Results are mean of 3 independent experiments with 20 sorghum seeds per experiment.
Error is within 5% limit. Cont. is control.
Abscisic acid (ABA,) is a plant growth inhibitor and indole butyric acid (IBA), is plant growth activator. At 100 ppm both ABA and IBA suppress the germination of wheat and sorghum seeds, although this effect is more pronounced in ABA rather than IBA, At 10 ppm germination percentage is 60-90% while at 1 ppm germination in IBA is >90% in both wheat and sorghum but ABA showed 75-90% germination. (Table 2)
|
Comp No |
3-ArylIndazoles |
3-ArylIndazole-1-acetic acids |
Yield (%) |
M. p. (oC) |
|
1 |
phenyl-1H-indazole |
3-Arylindazole-1-acetic acid |
82 |
165 |
|
2 |
3(2ʹ`-Hydroxyphenyl)- 1H-indazole |
3(2ʹ`-Hydroxyphenyl)indazole-1- acetic acid |
49 |
132 |
|
3 |
3-Styryl-1H-indazole |
3-Styrylindazole-1-acetic acid |
48 |
301 |
|
4 |
3(4ʹ -Acetaminophenyl)- 1H-indazole |
4-Acetaminobenzaldehyde |
29 |
201-3 |
|
5 |
3-(4ʹ -Chlorophenyl)-1H- indazole |
3-(4ʹ-Chlorophenyl)-indazole-1- acetic acid |
71 |
132 |
|
6 |
3-(2ʹ,4ʹ -Dichlorophenyl)- 1H-indazole |
3-(2ʹ,4ʹ-Dichlorophenyl)indazole- 1-acetic acid |
66 |
184-6 |
|
7 |
3-(4ʹ -Hydroxy-3ʹ- methoxyphenyl)-1H- indazole |
3-(4ʹ-Hydroxy-3ʹ- methoxyphenyl)indazole-1-acetic acid |
52 |
174 |
|
8 |
3-(3ʹ,4ʹ- Dioxomethylenephenyl)- 1H-indazole |
3-(3ʹ,4ʹ-Dioxomethylenephenyl)- indazole-1-acetic acid |
15 |
128 |
|
9 |
3-(2¢-Nitrophenyl)-1H- indazole |
3-(2ʹ-Nitrophenyl)indazole-1- acetic acid |
36 |
118 |
Table 1 Physical properties of 3-Arylndazole -1-acetic acids Derivatives.1-9
Effects on shoot length
Shoot length (mm) of developing wheat seedlings (Table 2) was measured on 5th day of germination. 3-Styrylindazole-1-acetic acid (3) and 3-(4ʹ-chlorophenyl)indazole-1-acetic acid (5) at 100 ppm completely inhibited germination of sorghum seeds (Figure 4) and no shoot and root growth was observed. Reduced shoot growth was observed with 3-(2ʹ,4ʹ- dichlorophenyl)indazole-1-acetic acid (6), 3-(4ʹ-hydroxy-3ʹ-methoxyphenyl)indazole-1- acetic acid (7) and 3-(3ʹ,4ʹ-dioxomethylenephenyl)indazole-1-acetic acid (8) while 3- phenylindazole-1-acetic acid (1) had small reduction in shoot length, 3-(2ʹ- hydroxyphenyl)indazole-1-acetic acid, 3-(2ʹ-nitrophenyl)indazole-1-acetic acid (9) exhibited shoot growth comparable with the control without any treatment (Table 2). At 10 ppm, some reduction in shoot growth was observed in 3-(4ʹ- acetaminophenyl)indazole-1-acetic acid (4). All other compounds like 3-phenylindazole- 1-acetic acid (1), 3-(2ʹ-hydroxyphenyl)indazole-1-acetic acid (2), 3-(4ʹ-hydroxy-3ʹ- methoxyphenyl)indazole-1-acetic acid and 9 demonstrated shoot lengths close to the control group which means no growth activation or inhibition activity but only compound 6 had growth stimulatory activity (Table 2). At 1 ppm, reduced shoot length was not observed and all had shoot length comparable to control. These results indicate that 3- (4ʹ-chlorophenyl)indazole-1-acetic acid (5), 6, 3-(4ʹ-hydroxy-3ʹ-methoxyphenyl)indazole- 1-acetic acid and 3-(3ʹ,4ʹ-dioxomethylenephenyl)indazole-1-acetic acid (8) at 100 ppm shoot growth inhibitors and showed up to 60% reduction in shoot growth.
|
Concentrations of compounds |
||||||
|
Compound |
100 ppm |
|
10 ppm |
1 ppm |
||
|
Root |
Shoot |
Root |
Shoot |
Root |
Shoot |
|
|
ABA |
0 |
0 |
9.1±0.2 |
10.5±0.7 |
14.5±1.8 |
12.8±1.3 |
|
IBA |
16.3±1.5 |
6.5±0.7 |
39.7±1.3 |
24±1.2 |
56±2.8 |
29±1.6 |
|
1 |
28±1.0 |
14±0.7 |
44±1.6 |
27±0.9 |
46±1.6 |
28±0.7 |
|
2 |
32±1.4 |
20±1.0 |
34±1.6 |
20±1.1 |
41±1.1 |
25±1.3 |
|
3 |
38±1.7 |
23±1.1 |
45±2.0 |
27±0.9 |
||
|
4 |
42±1.7 |
22±1.0 |
42±2.2 |
22±0.9 |
40±1.2 |
23±1.1 |
|
5 |
2±0 |
7±0.5 |
21±0.7 |
19±0.8 |
31±2.8 |
20±0.6 |
|
6 |
16±1.4 |
14±1.2 |
56±2.5 |
31±1.0 |
55±2.9 |
34±0.6 |
|
7 |
8±0.3 |
7±0.3 |
31±2.1 |
19±1.1 |
32±1.8 |
20±0.9 |
|
8 |
9±0.6 |
9±0.6 |
24±1.6 |
15±1.1 |
30±1.6 |
18±0.9 |
|
9 |
53±1.6 |
27±1.0 |
40±2.4 |
25±0.8 |
38±2.5 |
21±0.8 |
|
Control |
Root Length 34±5.3 |
|
Shoot Length 20±3.4 |
|||
Table 2 Effects of various concentrations of indazole-1-acetic acids on the root and shoot lengths (mm) on the 5th day of wheat seed germination. Results are expressed in terms of mean ± sem.
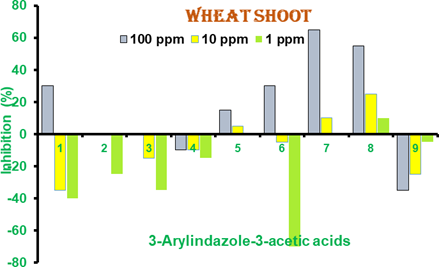
Figure 4 Percent inhibition of shoot length of wheat seedlings in 3- Arylindazole-1 acetic acids.1-9 Results are mean of 3- independent experiments. Positive bars are growth inhibitors while negative are growth activators.
In wheat seeds, absisic acid showed reduced shoot length at 10 and 1 ppm but at 100 ppm no increase shoot length was observed. While in sorghum seeds, reduced shoot length was observed at 100 and 10 ppm. On the other hand, in wheat, indole butyric acid at 10 and 1 ppm exhibited normal shoot length but at 100 ppm it reduced. In sorghum, ABA showed no particular effect on shoot length. (Table 3)
|
|
Concentrations of compounds |
|
|
||||
|
Compound |
100 ppm |
10 ppm |
1 ppm |
||||
|
Root |
Shoot |
Root |
Shoot |
Root |
Shoot |
||
|
ABA |
1.0±0.3 |
1.0±0.4 |
10±0.4 |
2.2±0.3 |
68.7±0.7 |
48.5±0.3 |
|
|
IBA |
30.8±3.4 |
3.5±0.4 |
61.5±3.3 |
40.8±4.5 |
82±4.3 |
49±4.4 |
|
|
1 |
46±0.9 |
31±0.9 |
39±1.6 |
41±0.6 |
41±1.0 |
53±1.0 |
|
|
2 |
48±1.2 |
41±0.8 |
48±1.1 |
49±0.4 |
51±1.5 |
46±1.7 |
|
|
3 |
- |
- |
35±1.6 |
32±0.9 |
10±0.1 |
13±0.8 |
|
|
4 |
10±0.3 |
11±0.6 |
54±1.0 |
60±0.7 |
42±1.2 |
41±0.9 |
|
|
5 |
20±0.3 |
21±0.3 |
24±0.9 |
28±0.6 |
15±0.6 |
14±0.3 |
|
|
6 |
23±0.5 |
14±0.4 |
55±1.4 |
52±1.1 |
43±1.3 |
47±1.1 |
|
|
7 |
26±1.5 |
24±0.8 |
42±1.3 |
44±1.0 |
35±0.7 |
39±0.7 |
|
|
8 |
15±0.3 |
23±0.4 |
35±0.5 |
38±0.3 |
42±1.2 |
39±0.6 |
|
|
9 |
41±1.2 |
43±1.3 |
44±1.1 |
48±1.0 |
56±1.6 |
51±1.0 |
|
|
Control |
Root length 52±4.7 |
|
|
Shoot length 49±3.9 |
|||
Table 3 Effects of various concentrations of indazole-1-acetic acids on the root and shoot lengths (mm) on the 5th day of sorghum seedling growth after germination. Results are expressed in terms of mean ± sem.
Effects on root length
Changes in root length (mm) were monitored on 5th day of wheat seed germination Root growth was inhibited at various levels at various concentrations. 3-(4ʹ- Chlorophenyl)indazole-1-acetic acid (5), 3-(2ʹ,4ʹ-dichlorophenyl)indazole-1-acetic acid (6), 3-(4ʹ-hydroxy-3-methoxyphenyl)indazole-1-acetic acid and 3-(3ʹ,4ʹ- dioxomethylenephenyl)indazole-1-acetic acid (8) exhibited maximum inhibition of root growth at 100 ppm but 3-(2ʹ-nitrophenyl)indazole-1-acetic acid (9) had root growth induction effects. At 10 ppm, root length was inhibited by 3-(4ʹ-chlorophenyl)indazole- 1-acetic acid (5), 3-(3ʹ,4ʹ-dioxomethylenephenyl)indazole-1-acetic acid (8) and 3-phenylindazole-1-acetic acid (1), 3(4-acetaminophenyl)indazole-1-acetic acid (4), 3- (2ʹ,4ʹ-dichlorophenyl)indazole-1-acetic acid (5) and 3-(2ʹ-nitrophenyl)indazole-1-acetic acid (9) demonstrated stimulation of root length. Rest of the compounds at this concentration showed root growth comparable to that of control. At 1 ppm, no compound showed reduction in root growth rather some compounds like 3- phenylindazole-1-acetic acid (1), 3-styrylindazole-1-acetic acid (3), 3-(4ʹ- acetaminophenyl)indazole-1-acetic acid (4), 5 acted as root growth stimulators (Table 2).
Changes in root length (mm) in sorghum seedlings were monitored on 5th day of germination. All compounds reduced root growth by various extents but 3-(4ʹ- acetaminophenyl)indazole-1-acetic acid (4), 3-(4ʹ-chlorophenyl)indazole-1-acetic acid (5), 3-(2ʹ,4ʹ-dichlorophenyl)indazole-1-acetic acid (6), 3-(4ʹ-hydroxy-3ʹ- methoxyphenyl)indazole-1-acetic acid (7), 3-(3ʹ,4ʹ-dioxomethylenephenyl)indazole-1- acetic acid (8) were the most effective inhibitors of root growth at 100 ppm compared with the control. However, root growth was induced by 20-40% at this concentration by 9. Similar profiles were observed at 10 ppm. All compounds 3-styrylindazole-1-acetic acid (3) 3-(3ʹ,4ʹ-dioxomethylenephenyl)indazole-1-acetic acid (8) inhibited root growth at variable extent and compounds had no effect on root growth compared with the control (). At 1 ppm 3-styrylindazole-1-acetic acid (3), 3-(4ʹ-chlorophenyl)indazole-1-acetic acid (4) and 7 was inhibitor of root growth while all other compounds showed moderate growth comparable to control (Table 3).
In case of wheat no root length was observed at 100 ppm by ABA but at 10 ppm some what reduced root length was observed while at 1 ppm root length observed compared to untreated water or control. In sorghum seeds, at 100 ppm ABA showed extremely reduced root growth and at 10 ppm reduced root length was observed while at 1 ppm no significant difference was observed with root length of untreated control (Table 2, Figure 2). IBA at 100 ppm exhibited reduced root growth while at 10 ppm normal root length was observed but at 1 ppm root length was increased.
Percent Inhibition/Stimulation of Growth
When percentage values of test compounds were calculated for wheat taking untreated control as 100 percent, it was found that in wheat none of the compounds were growth accelerators. 3-Styrylindazole-1-acetic acid (3), 3-(4ʹ-acetaminophenyl)indazole-1-acetic acid (4), 3-(2ʹ,4ʹ-dichlorophenyl)indazole-1-acetic acid (6) are found as good inhibitors of growth. 3-(4ʹ-Chlorophenyl)indazole-1-acetic acid (5), 3-(4ʹ-hydroxy-3ʹ- methoxyphenyl)indazole-1-acetic acid (7), 3-(3ʹ,4ʹ-dioxomethylenephenyl)indazole-1- acetic acid and 3-(2ʹ-nitrophenyl)indazole-1-acetic acid (9) showed normal growths with no particular effects (Figure 6).
In sorghum, 3-styrylindazole-1-acetic acid (3), 3-(4ʹ-acetaminophenyl)indazole-1-acetic acid (4), 3-(2ʹ,4ʹ-dichlorophenyl)indazole-1-acetic acid (6) clearly proved growth inhibitors and 4 was found as activator of roots (Figure 4) and shoot growth (Figure 6). 3- (3ʹ,4ʹ-Dioxomethylenephenyl)indazole-1-acetic acid (8) and 3-(2ʹ-nitrophenyl)indazole-1- acetic acid (9) had neither inhibiting nor activator effects on growth.
Indazole-1-acetic acids are also biologically important compounds and have versatile biological activity. These are inhibitors of aldose reductase enzyme and used as anti- diabetic agents. But Yusuo F4 reported the effect of 5-chloro-1H-indazoleacetic acid on fruit thinning of Satsuma mandarin. When 100 or 200 ppm 5-chloro-1H-indazole-3- acetic acid was sprayed 40-50 days after full bloom Satsuma mandarin showed excellent thinning performance especially for dropping the small and young fruits and did not cause injury on the fruits and leaves. The increase of total sugars and the decrease of citric acid during development were hastened by the application of 5-chloro-1H- indazoleacetic acid on fruit weight increase was less than by the application of 300 ppm.
In our experiment indazole-1-acetic acids are inhibitor at high concentration of 100 ppm and while at 10 and 1 ppm the effect is activator instead of inhibition. In sorghum activation is much important at 1 ppm especially in case of 3-phenylindazole-1-acetic acid (1) and 3-(2ʹ,4ʹ-dichlorophenyl)indazole-1-acetic acid (6) the activation is much higher. In case of sorghum there is only inhibition of both root and shoot. No activation was observed in sorghum. 3-Styrylindazole-1-acetic acid (3), 3-(4ʹ- chlorophenyl)indazole-1-acetic acid (4) and 3-(3ʹ,4ʹ-dioxomethylenephenyl)indazole-1- acetic acid (8) are inhibitors at all concentrations for both root and shoot. In indazoles shoot inhibiting effect was more pronounced as compared to root length inhibition. (Figure 5 & 7)
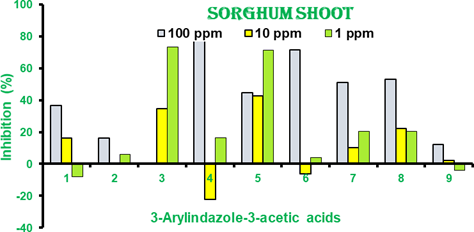
Figure 5 Percentage inhibition of shoot length of sorghum seedlings in 3- arylindazole-1-acetic acids (1-9) . Results are mean of 3- independent experiments. Positive bars show growth inhibition while negative bars as growth activation.
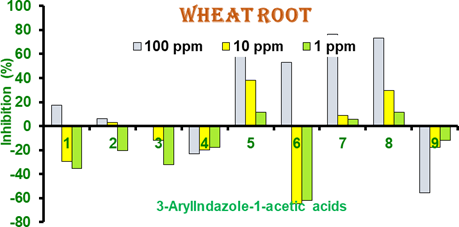
Figure 6 Percent inhibition of root of wheat seedlings in 3-Arylindazole- 1-acetic Acids.1-9 Results are mean of 3-independent experiments. Positive bars are growth inhibitors while negative are growth activators.
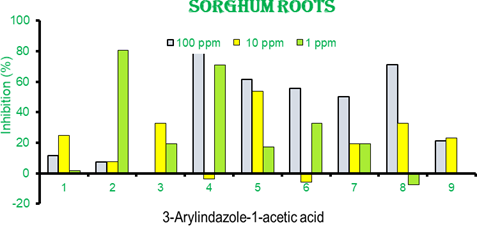
Figure 7 Percentage inhibition of root length of sorghum seedlings in 3- arylindazole-1-acetic acids.1-9 Results are mean of 3- independent experiments. Positive bars show growth inhibition while negative bars as growth activation.
According to Iwata et al.12 it played an important role in thinning of young fruits and blossoms when applied to fruit trees. A study on different indazole-3-acetic acids and their derivatives have shown that 5-chloro-1H-indazole-3-acetic acid, R = H, X = Cl) and ethyl 5-chloro-1H-indazole-3-acetate (R = ethyl, X = Cl) are more potent and active ingredient. Seed growth inhibition is more pronounced in indazole-1-acetic acids in our study.
Indazoleacetic Acids Derivatives X= Cl, F, R= H, CH3, C2H5
Takashi et al.13 described indazole as plant growth regulator. The indazole derivatives I (X and Y = H or Cl, R = CN or COOH) are plant-growth especially suited as rooting stimulators for cuttings. 6-chloro-3-indazoleacetic acid is an example of plant growth regulator. Kazu et al.14 described the indazole actetaes. Indazoleacetic acids (R = H, halo), useful as plant growth regulators were prepared.
According to our observation indazole-1-acetic acids are inhibitor of plant growth at high concentration as 100 ppm and moderate inhibitor at 10 ppm but it effect becomes activator at low concentration as 1 ppm. So, indazole-1-acetic acids can be used as both as inhibitor and activator at different concentrations.
It is concluded that 3-arylindazole-1-acetic acids are supposed to delay the germination of seeds simply by lowering the metabolic process during the imbibitions hence can be useful for seed storage. These derivative can be used as early growth accelerator of planlets. Indazoleacetic acids can be used as growth accelerators of wheat and sorghum seedling in contrast to its respective 3-aryl-1H-indazole derivatives which are plant growth inhibitors.
None.
The authors declare that there is no conflicts of interest.

©2024 Chattha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.