eISSN: 2576-4462


Literature Review Volume 4 Issue 3
1Posgradraute College Campus Córdoba, Veracruz, México
2Posgradraute College Campus Montecillo, Estado de México, México
Correspondence: Josafhat Salinas Ruiz, Posgradraute College, Campus Córdoba, Amatlán, Veracruz, México, Tel 2717166000
Received: May 05, 2020 | Published: May 11, 2020
Citation: Castillo-Herrera N, Hidalgo-Contreras JV, Vequia HDD, et al. Bibliometric research of technology used in harvest and postharvest of papaya. Horticult Int J. 2020;4(3):68?73 DOI: 10.15406/hij.2020.04.00160
Papaya is a tropical fruit of economic, industrial, medicinal and nutritional importance, produced in 70 countries. The objective of this research was to carry out a compilation and analysis of the information related to harvest maturity indexes, shelf life, main diseases and post-harvest treatments of papaya, for which the VOS viewer software was used. It is possible to determine a harvest index by correlating the color with the internal physicochemical characteristics of the papaya. The short shelf life is attributed to the physiology of the fruit and the susceptibility to diseases such as anthracnose. Derived from this, the investigations carried out in the last 10 years have focused on technologies such as; edible films and coatings with various antifungal compounds; compounds that delay fruit ripening; and post-harvest treatments to extend shelf life.
Keywords: maturity index, shelf life, diseases, post-harvest treatments, films, edible coatings, anthracnose.
FAO, food and agriculture organization; 1-MCP, 1-methyl cyclopropene; CIEL*a*b*, L*, brightness, a*, value a, b*, value b; PG, polygalacturonase; PME, pectin methyl esterase; TSS, total soluble solids; CONACyT, national council for science and technology
Papaya (Carica papaya L.) is a tropical, climacteric fruit, known worldwide for its nutritional benefits. World production in 2018 was 13290 320 t ∙ yr-1. This fruit is produced in 70 countries, in Asia more than 57% and in America 31%. India is the main producer with 5989000 t ∙ yr-1, Brazil second with 1060392 t ∙ yr-1 and Mexico in third place with 1039820 t ∙ yr-1. Mexico is also the main exporter of papaya in the world.1
Papaya is a fruit with a high nutritional content, practically the entire plant has nutritional and medicinal properties. In the fruit, the nutritional content varies according to the state of maturity. In the green state, the fruit has a high content of vitamin C and total phenols, therefore its antioxidant capacity is greater when it is green. In the mature state, the fruit has a high concentration of beta-carotenes.2 The medicinal properties of papaya have attracted the attention of many researchers due to its antioxidant, antimicrobial, anti-inflammatory and anticancer qualities.3
In addition to the nutritional and medicinal uses of papaya, industrial uses of this product have also been studied. Papaya contains an enzyme known as papain, which is used in the food industry as a meat tenderizer, in the textile industry it is used to soften skins. It is also used in the pharmaceutical industry.4 In accordance with the aforementioned, papaya is a worldwide consumed for its nutritional, medicinal and industrial qualities. Therefore,5 this product has had an increasing demand in the world and, so, a greater production and export.6
Papaya has positioned Mexico as a quality exporter. However, Mexico still has a very wide gap, with respect to other countries, in the implementation of new technologies that contribute to maintaining product quality and therefore reducing losses in the production and marketing process. Diseases, bad agricultural practices and the physiology of the fruit are factors that most affect quality.7 One of the biggest challenges in papaya production is to extend its shelf life.
In the last decade, various researchers have generated very important information on technologies that seek to extend the shelf life of the fruit and reduce post-harvest losses due to over-ripening and diseases. The objective of this research was to carry out a compilation and analysis of the information related to harvest maturity indexes, shelf life, main diseases and post-harvest treatments for papaya, by using the VOSviewer software.
Analysis of information on papaya
To visualize graphically the investigations carried out in the last 10 years on papaya, two maps were created in VOS viewer from 1086 bibliographic data obtained from Scopus. The filters of the year 2010-2020 and keywords such as papaya, post-harvest technologies, ripening and diseases were used to delimit the search area for Scopus.
The first map is a network made up of themes. VOSviewer classified eight groups according to the co-occurrence between the titles of the articles; the second map is a network formed by links between authors, the network is made up of 10 groups classified by co-authorship. Figure 1 is a map showing the papaya harvest and post-harvest research topics. The most outstanding research topics on this map are different varieties of papaya that are included in the group of tropical fruits, genetics and the physicochemical characterization of papaya.
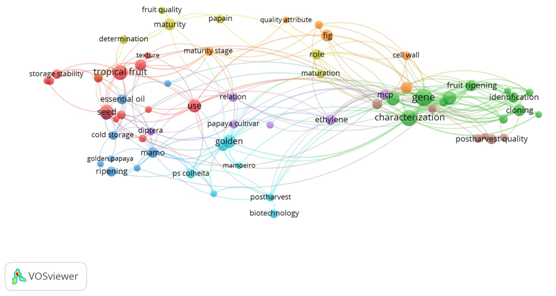
Figure 1 Map of publications on papaya. The size of the circle and the label indicate the importance of the topic (bigger size bigger weight). The color of each element is based on the group to which it belongs.
The use of compounds to extend the shelf life of the product is another of the topics that have been investigated most frequently, such as 1-methylcyclopropane (1-MCP), which works as an inhibitor of the action of ethylene. In this network, it can be seen that in four of the eight groups the term maturity or ripening is mentioned, this means that most of the investigations are related to the biochemical processes that occur during fruit ripening.8,9
The group least related to the other groups is postharvest quality. This does not mean that post-harvest quality is not being investigated, it is just that those words were less used in the titles of the papers. However, most research aims to maintain the quality of the fruit. For example, various treatments that can be viewed on the map; cold storage; use of essential oils in films and coatings; and the action of the 1-MCP.
Figure 2 shows that the author with most articles published on papaya is Paull R. E. with 28 papers followed by Kanlayanarat S. with 22 and in third place is Fabi J. P. with 15 articles. Of these authors, the most cited is Paull R. E. with 709 citations and Lajolo F.M. with 651 citations. The co-authorship force joins to six groups on the right side and four on the left side, where the only connection between them is between Chen W. and Paull R. That is, the author with the most collaborations is also Paull.
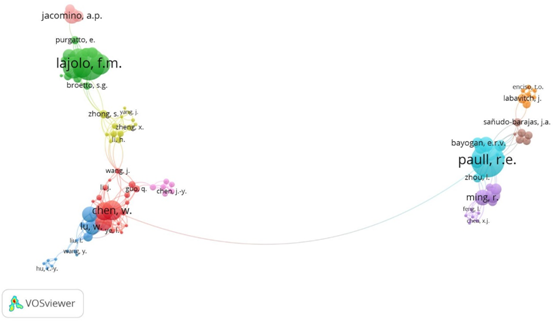
Figure 2 Map of authors who publish related topics on papaya. The size of the circle and the label indicates the importance of the topic. The color of each element is based on the group to which it belongs.
Harvest maturity indices
The maturity of a fruit is a process given by a series of physiological, biochemical and organoleptic changes. For example; chlorophyll degradation; enzymatic degradation; the formation of aromatic compounds; increased sugar content; respiration and ethylene production.10 The ripening of papaya directly affects the sensory characteristics of the fruit such as taste, smell, aroma, texture, etc., as well as nutritional quality. Therefore, it is important to take care that the fruit ripens properly. The main harvest indicator for papaya producers is color. This subjective harvest index is based on the color change in the epidermis of the fruit, from light green to yellow.
Some authors have proposed harvest indicators based on correlation of the external color of the fruit with the internal physicochemical characteristics. The degree of maturity of papaya can be measured with help of a model that considers color as the most important variable. That is, the physicochemical properties such as firmness, respiration, total soluble solids, pH and acidity can be correlated with color and determine the state of maturity.11
Color
The color of a fruit is a function of the physicochemical, microbial, and biochemical reactions that occur inside the fruit during ripening in the pre and post-harvest processes and in storage.12 The color change from green to yellow is due to the decrease in chlorophyll content and the production of carotenoids, lycopene and other pigments that give papaya its characteristic color.11
The color of the fruits has generally been obtained through use of colorimeters in the CIELAB space. This scale is a standard for comparing colors and is therefore considered reliable.13 In papaya, color measurements on the CIEL * a * b * scale have revealed that the luminosity values and the chromatic coordinates a* and b* increase linearly as the state of maturity of the papaya progresses.1
The colorimeter is a very practical instrument, this makes it widely used. However, some authors point out that these measurements may not be adequate, because the color of the papaya epidermis is heterogeneous, that is, there are color variations that may not be taken into account when only measuring some points. Although, there are other methods that could be more accurate for measuring heterogeneous color, such as digital images,14 fractal metrics and image texture.13
Firmness
The softening of the pulp in the papaya fruit is one of the most notable changes that occur during the ripening process.15 These changes are due to hydrolysis of polysaccharides present in cell wall of the fruit, resulting in a modification in its firmness.
Loss of firmness is less in the first days after harvest but as the ripening process progresses, the morbidity of fruit increases as increase enzyme activity in conjunction with respiration and ethylene production.10 This enzymatic activity is mainly due to two enzymes; polygalacturonase (PG) and pectin methyl esterase (PME).16
The firmness of the papaya mesocarp is related to the color of the fruit, that is, an increasing of yellow and reddish tones in its epidermis, the fruit loses firmness. Therefore, this color change is perceptible when handling the fruit and it could be considered only as an indicator of organoleptic maturity; however, to determine the physiological maturity this change of color could be a bad indicator and it could compromise fruit quality in marketing.
The firmness of papaya is one of the greatest challenges, especially during storage, because the softening of the fruit is related to its senescence. In this sense, various researchers have proposed treatments to delay loss of firmness of the fruit such as doses of ozone less than 5 ppm,17 calcium chloride and citric acid in combination with modified atmosphere packaging at 5oC,18 and coating based on sodium alginate and cellulose acetate.19
Respiration
The respiration rate is an important parameter to consider in the post-harvest, since papaya is a climatic product whose respiration rate and ethylene production are positively correlated with its maturity.20 The main gases that are produced during respiration process are O2, CO2, and C2H4. The respiration process if this fruit is influenced by ripening process, its physiology of the fruit and external conditions such as temperature and atmosphere.21
Total soluble solids (TSS)
The TSS are an indicator of the sugar content in the fruit. TSS in combination with other compounds are responsible for the characteristic flavor and aroma of papaya.22 The soluble sugars contained in the pulp of the papaya are sucrose, glucose, and fructose. The SST increase linearly and gradually as the ripeness of the papaya advances. As the color of the fruit turns yellow, the TSS increases.
In the Maradol variety, the TSS content range from 8.19% in its physiological maturity and can reach up to 12.87% when it is fully mature. This occurs approximately in 158 days after anthesis. In the Tainung variety, researchers have reported a content of SSTs of 9.25 and 11.09% in papayas with 65 and 85% maturity, respectively.13 On the other hand, Golden papaya can reach 13.4 °Brix during its organoleptic maturity.23
pH and titratable acidity
The pH and the titratable acidity vary during the maturation process. Both parameters tend to increase or decrease depending on the post-harvest treatment given to the fruit, even some authors report insignificant changes during ripening.8 Variations in pH and titratable acidity are due to the conversion of organic starches and acids to sugars through catabolic degradation and glycogenesis.10
The pH values tend to increase slightly during ripening but do not exceed the neutral value, giving the slightly acidic flavor of papaya. At physiological maturity, the pH ranges between 4.32 - 4.75 but we can see measurements of 5.55 - 5.79 in an advanced stage of maturity.
Titratable acidity also increases as fruit maturity progresses. During organoleptic maturity, a higher concentration of citric acid is reached, so there can be a significant difference in titratable acidity between the first stages of maturation and the optimal consumption.11
Shelf life
The shelf life of papaya is short because it is a climacteric fruit and susceptible to disease.24 However, the deterioration of the fruit could be delayed if conservation treatments are carried out during its pre and post-harvest.25 There are technologies in post-harvest management of other fruits that are difficult to extrapolate to management of the papaya fruit, due to the fact that their genetic characteristics limit it.26
The shelf life of papaya depends largely on post-harvest treatments, storage, packaging, and transportation. A papaya in tropical climate conditions and without any treatment can have a shelf life of approximately seven days.20
Films and coatings
Films offer a modified atmosphere to the fruit that can delay biochemical processes during ripening.9 This is because they reduce water loss, decrease the respiration rate, as well as avoid the direct contact with organisms that damage the fruit.27 Some of the compounds that have been reported in the use of films are papaya puree with defatted soy protein and gelatin;28 pectin in combination with aqueous and alcoholic extracts of acerola (Malpighia emarginata);29 and papaya peel micro particles as an antioxidant ingredient.30
Coatings as well as films seek to preserve sensory properties of the fruit as long as possible.9 Coatings made of various materials have been reported like sodium alginate and cellulose acetate;19 papaya leaf extract with aloe vera gel,31 aloe vera gel and honey with chemical immersions,32 cassava starch,33 ginger oil and gum arabic,24 Aloe gel with chitosan and essential oils,27 and UV-C radiation and chitosan with essential oils.34 All these combinations of compounds in films and coatings can be an alternative to reduce the use of fungicides and synthetic packaging.
Postharvest diseases and treatments
Postharvest diseases caused by pathogens affect the quality of papaya during the postharvest process. One of the diseases that most affects papaya is anthracnose. This disease is caused by a fungus called Colletotrichum gloeosporioides.35 Losses of up to 50% have been reported in products sold fresh due to this disease,36 even some wholesalers report losses of up to 75% in post-harvest in papaya. This could be due to genetic characteristics of the papaya and the ripening process make it very susceptible to injury or damage caused by handling.26 The most studied treatments are chemical, thermal and based on natural extracts. The use of only type of treatment has had little success in combating diseases, so the use of combined treatments has been the best option.34
Treatments using chemical ingredients
Chemical fungicides are the most used to fight diseases due to their high effectiveness and low cost.25 Prochloraz and Propiconazole-based fungicides have shown their effectiveness for that reason they are widely used to combat anthracnose.37 The immersion of papayas in cinnamaldehyde with calcium chloride has been effective in protecting the fruit against Salmonella and Escherichia coli.38
Ayón ‐ Reyna et al.39 reported the effectiveness of hot water and a subsequent application of calcium chloride against Colletotrichum gloeosporioides in papaya fruits. Supapvanich & Promyou40 mention that, the use of hot water prior to the application of salicylic acid is an effective treatment to control the incidence of fungi in papayas during storage.
Moreover, hot water treatments have been effectively combined with irradiation to decrease incidence of fungal infections in the epidermis of papaya.41 However, they are treatments where you must be very careful with the application. In this regard, Asgar Ali et al.24 mention that subjecting papayas to heat treatment can affect nutritional and sensory properties as well as the ripening process.
Other alternatives are ozone treatments, which have shown to be effective in fighting diseases in papaya. A dose of 1.6 ppm of ozone delays the appearance of anthracnose for up to 21 days.37 Likewise, the exposure of papaya fruits to gaseous ozone in doses of 2.5 ppm for 96 hours maximizes the nutritional properties of antioxidant activity, beta-carotene content, ascorbic acid content and the concentration of sugars. However, if the level of 2.5 ppm ozone is exceeded, there could be damage to the aesthetics of the fruit due to its strong oxidizing activity.17
Treatments using natural ingredients
The use of some fungicides has been restricted due to their toxicity in human health and their slow degradation in the environment. Furthermore, the indiscriminate application of agrochemicals creates resistance in fungi and bacteria that cause the diseases.42 For this reason, researchers have been looking new technologies with natural and efficient ingredients that maintain quality of the fruit.43
Essential oils have been studied for their antimicrobial and antifungal properties. These oils are secondary metabolites that aromatic plants produce as self-defense and have the ability to fight pathogens. The essential oil of liquid and steam lemongrass (Cymbopogon) have been used in papayas to reduce the development and growth of the Colletotrichum gloeosporioides, without affecting maturation.44
The use of castor oil (Ricinus communis) has also been suggested as an anthracnose inhibitor in papaya.42 Likewise, the essential oil of satureja (Satureja khuzistanica) and thyme (Thymus daenensis) have been effective in controlling the mycelial growth of the fungus that causes anthracnose.45
Biological control methods are also studied as an alternative to fight disease. Some of the organisms used successfully as biological control are Pseudomonas syringae, Bacillus subtilis, Candida oleophila, Cryptococcus albidus and Streptomyces griseoviridis.25 The use of Bacillus amyloliquefaciens PPCB004 in combination with 1-methylcyclopropane reduces significantly the incidence of disease and extends the shelf life of papaya. This last treatment could be used in the market of organic products.46
The growing demand for papaya worldwide is due to the nutritional characteristics that this fruit provides. Papaya is a climacteric fruit whose shelf life is short, due to its physiology. This fruit is very susceptible to diseases, especially anthracnose, leading to post-harvest losses of up to 50%. Research carried out in the last decade proposes technologies that seek to extend the shelf life of papaya by developing harvest indices based on the correlation of the color of the epidermis with other physicochemical characteristics; combination treatments to control diseases; edible films and coatings.
Thanks to Posgradraute College Campus Córdoba and The National Council of Science and Technology (Conacyt) for facilities provided to carry out this work.
Authors declare no conflict of interest exists.

©2020 Castillo-Herrera, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.