eISSN: 2576-4462


Research Article Volume 7 Issue 4
Department of Plant, Food, and Environmental Sciences, Faculty of Agriculture, Dalhousie University, Bible Hill B2N 5E3, Nova Scotia Canada
Correspondence: Sparsha Chada, Department of Plant, Food, and Environmental Sciences, Faculty of Agriculture, Dalhousie University, Bible Hill B2N 5E3, Nova Scotia Canada, Tel +91-799-312-3532
Received: November 01, 2023 | Published: November 14, 2023
Citation: Chada S, Asiedu SK, Ofoe R, et al. An overview of plant morpho-physiology, biochemicals, and metabolic pathways under water stress. Horticult Int J. 2023;7(4):115-125. DOI: 10.15406/hij.2023.07.00285
Water stress poses a significant risk to achieving global food security. Plants experience water stress due to variations in environmental conditions, which have been identified as the primary factor impacting agricultural production under the current dispensation of global climate change. This review aims to combine data from multiple published research studies in the literature in order to completely analyze and assess the morphological, physio-biochemical, and metabolite responses of plants to water stress. Stress-related variables disturb the regular stability of plants, leading to changes in their structure, function, chemistry, and genetics that ultimately impact their development and productivity. Water stress often leads to reduced leaf-relative water content, loss of turgor, and closure of stomata. Plant secondary metabolites serve as distinct sources for medicines, food additives, and industrially essential biochemicals. Plants exposed to water stress, such as different elicitors or signal molecules, frequently experience the build-up of these metabolites. Secondary metabolites significantly contribute to plant ability to adapt to their environment and overcome stressful conditions. Plants exhibit diverse metabolic reactions to water stress, resulting in alterations in the composition of volatile oils in different herbs. The attempt to tackle water stress conditions by plants is inherently intricate due to the complex network of signaling. This review contributes to our understanding of plant responses to water stress conditions, to the implementation of sustainable agronomic practices, and development of varieties that can tolerate the impact of climate change.
Climate change is posing a significant threat to life on Earth. In the present fluctuating climatic circumstances, fulfilling the increasing food demand and attaining a sustainable agri-food system for a growing population is becoming difficult.1 Among the abiotic stresses, water stress is the most detrimental that hinders agricultural productivity to a great extent worldwide.2 Generally, plants can endure water stress, but at a significant cost to total biomass production. About half of the semi-arid and arid regions of the world are affected by water stress, which occurs owing to either water surplus or water scarcity. The entire area of the world's dry lands has expanded considerably in the last decade, and the extent of drought has resulted in USD 30 billion of global crop losses annually.3,4 Additionally, the intensity and frequency of flooding disasters have been on the rise since the 1990s.5,6 For instance, the floods caused a total crop loss of USD 5.5 billion between 1982 and 2016.7 The World Resource Institute (WRI) Aqueduct water risk atlas reveals that approximately 25 countries experience exceptionally severe water stress annually, consistently depleting nearly all of their accessible water resources. Approximately 4 billion individuals, which is equivalent to at least 50% of the global population, reside in areas characterised by severe water stress.8 (Figure 1)

Figure 1 World Resource Institute (WRI) Aqueduct water risk atlas shows that about 25 countries accommodating one quarter of the global population is highly under water stress conditions (Institute, 2023).
Water stress interrupts the regular functions of plants leading to morphological, physiological, and biochemical alterations (Figure 2).9,10 Previous research revealed that water stress induces oxidative stress, which negatively impacts biological membranes and macromolecules (DNA, proteins, lipids, and photosynthetic pigments).11,12 Water stress leads to nutritional imbalances, which have a large ecological impact on global agricultural output.13 However, most plants possess a well-studied innate system for resisting water stress, which includes water stress-resistant epigenetic plasticity and gene activation, as well as morphological, physiological, and biochemical adaptations.14

Figure 2 Impact of water stress on the structural, functional, and metabolic components of plants (mofified from Wahab et al., 2022)).
This review focuses on the effects of water stress on plant morphology, physiology, and biochemical processes. Also, it explores the response of plants to alterations in central carbon metabolic pathways. These are crucial elements for understanding how plants respond to water stress. The understanding of these mechanisms under water stress is vital for boosting plant output under challenging conditions.
The changes in precipitation patterns observed currently can be attributed to global climate change, as demonstrated by increasing temperatures and heightened atmospheric CO2 concentrations.15-18 The primary trigger for water stress on a global scale is the sustained global climate change.19 Nevertheless, water stress can be caused by other factors including elevated temperatures, intense sunlight, and arid winds, all of which contribute to the accelerated evaporation of soil moisture.20 Furthermore, these variables contribute to elevated water losses from plants, hence promoting plant vulnerability to water stress. Occasionally, drought does not arise solely from a scarcity of water in the soil but also, due to several soil conditions such as soil texture and structure, salinity, soil temperatures, barrier to absorption of water by roots, and diseases, despite the presence of sufficient water in the soil.21 This particular form of drought is referred to as pseudo-drought or physiological drought, and it is not influenced by climatic conditions.18 Floods on agricultural land typically result from either heavy or prolonged precipitation, however, they can also arise when water bodies overflow onto the land.22
The key indicators of how plants are affected by water stress can be observed in their leaves and roots, which exhibit apparent changes in their external morphological characteristics and interior anatomical structure. Water stress adversely affects early germination, plant height, relative root length, root diameter, the total biomass of leaves and roots, number of leaves and canopy size per plant.
Early seed germination and flowering
Seed germination is the initial and critical phase of a plant development.23 Water is crucial for the process of seed germination because even if all other conditions are met, water stress prevents seeds from absorbing water and that can impede germination.24 Water stress directly impacts seed water imbibition, which results in decreased seed germination rate and inadequate seeding establishment.25 In order to achieve optimum seed germination, the soil must contain adequate moisture to enable the initiation of metabolic processes, the breaking of dormancy, and the transformation of stored nutrients in the seed into forms that can be utilized by the embryo.26 The impact of water stress on plants might vary according to the duration, severity, and phenological stage. Once the seeds ingest water, the process of metabolic modifications are initiated.23 The availability of soil water and its water potential is the primary parameters that influence the absorption and utilization of water during the initial stages of germination.27 Furthermore, dry conditions during the initial period greatly hinder the process of seed germination and seedling establishment. This is mostly due to a decrease in water absorption during the germination phase, a reduction in energy supply, and a weakening of enzyme activities. Seeds undergo a loss of viability and vigor throughout the process of dehydration.23 This phenomenon arises due to numerous metabolic alterations encompassing the generation of reactive oxygen species (ROS) that inflict harm upon RNA, DNA, and proteins leading to disruption of membrane integrity, diminished respiratory activity, and reduced ATP synthesis.27
Plant morphological structures exhibit specific alterations to water stress
The impact of water stress on plants is primarily evident in their root and shoot systems. The most accurate reflection of the ability of the roots and leaves to adjust to unfavorable conditions is through the assessment of their exterior morphological traits and internal anatomical structure.28-30 Compared to roots, leaves exhibit the highest degree of variability in their long-term adaptation to the environment. Leaves exhibit similar responses to both drought and waterlogging stress, showing symptoms such as etiolation, atrophy, curling, senescence, and even abscission.31-32 Studies have shown that water stress can inhibit leaf growth and reduce leaf count and size.4,33,34
Shoot: A primary consequence of water stress is a reduction in plant growth as a result of less photosynthetic activity. Stem growth and in particular, leaf growth exhibit greater susceptibility to water stress compared to root growth. During periods of water stress, the plant reduces the rate of stem elongation and stimulates root growth as a response to seek additional water resources.35 Drought can restrict plant growth by impeding the division of cells in leaf meristematic tissues and the enlargement of cells in elongation regions. Additionally, it can trigger intricate alterations in leaf thickness, palisade tissue, and spongy tissue as part of the adaptation process.36-38 The primary physiological consequences of leaves in response to waterlogging stress include leaf curling, yellowing, wilting, abscission, and decay. Leaves exhibit two types of adaptations to waterlogging stress: one augment their thickness, while the other diminishes their thickness.4 To achieve the desired outcome of the reduction of water loss and enhancement of water retention capacity, it is necessary to increase the amount of palisade and spongy tissues while simultaneously decreasing the size of leaves and stomata.39-41 The latter occurs because leaves are unable to undergo normal morphogenesis as a result of insufficient water and nourishment.42 Consequently, certain plants adjust their leaves by either reducing their thickness to enhance the capacity for CO2 and inorganic nutrients to enter the leaves. This adaptation mechanism will facilitate improved gas exchange, enabling the plants to sustain respiration even in conditions of waterlogging stress.43
Root: Roots modify their morphology in order to enhance their ability to penetrate, spread, and make contact with the soil, hence facilitating more efficient absorption of water and nutrients in response to water stress conditions.44-45 These structural changes guarantee the essential intake of nutrition and water, hence sustaining plant physiological functions and productivity in times of water stress.46 During periods of drought, plants experience increased expenditure of photosynthates. Typically, plants distribute photosynthates to develop root systems in order to seek water and nutrients necessary for metabolic processes.46 It is important to allocate more resources towards root growth in order to enhance water absorption. Additionally, plants raise their respiration rate to sustain the roots in the drying soil. As a result, these mechanisms due to water stress compromise the overall productivity of the plant.47-48 The lack of water in the soil leads to an increase in mechanical resistance, which limits the ability of roots to penetrate into the deeper layers of the soil. This hampers the plant ability to utilize resources, resulting in a decrease in agricultural output.50
Anatomical adaptation enhances root penetration in arid soil. For instance, smaller outer cortical cells serve to stabilize the root by preventing ovalization and collapse, and facilitating soil penetration. On the other hand, the presence of large cortical cells in the mesodermis and the presence of thick axial roots with abundant aerenchyma serve to reduce the energy expenditure associated with soil exploration, and facilitate root growth in compacted soils.50 Hydrotropism, which refers to the directional growth of plant roots towards moisture, is crucial for plants to adapt to drought conditions. Root tips exhibit hydro patterning, wherein they counteract the effects of varying water potentials by adjusting their development direction to branch towards places with increased water availability regardless of gravitational forces.51 Hydrotropic growth aids the movement of developing lateral roots towards water when they are surrounded by dry soil. Hydrotropism and hydropatterning work together to help plants adapt to drought conditions.52
The impact of water stress on plant growth and development
Drought can significantly impair plant growth and development.53 Drought is a complex form of stress for plants that can affect all aspects of their growth and development.21 Furthermore, drought can have adverse effects on both the quantity and quality of plant development and productivity.54 Cell division, elongation, and differentiation are crucial factors that determine the growth and development of plants. Drought lead to a decrease in plant water potential and turgor, hence, impairing the ability of plant cells to carry out their usual duties.55 Thus, drought impact all of the phases of plant growth and development starting from a decrease in cell turgor, disruption of enzyme activities, and a reduction in energy supply from photosynthesis.56 Cell proliferation and enlargement are essential processes during the early stage of plant development and establishment. Plant response to water stress and return to normalcy varies with the intensity of stress, and the length and timing of stress exposure in addition to the inherent health of the plant.21
Water stress has been reported to cause physiological stress in plants, and although different plants may respond differently, water stress frequently results in decreased leaf-relative water content, turgor loss, and stomatal closure.57 (Figure 2)
Stomatal aperture
Stomata closure is the foremost indicator of a plant response to water stress. Plants adapt to water stress by controlling stomata movement, altering osmotic balance, and initiating an antioxidant defense system.58 In periods of intense water stress, stomata are completely closed in most plant species.59,60 Water stress tolerance plants influences the rates of carbon fixation, photosynthesis, and water use efficiency since the stomatal system governs these processes.57 Stomatal closure restricts CO2 entry, allowing the electrons to produce more reactive oxygen species (Figure 2).61 Studies have shown that water stress decreases stomatal conductance due to reduced aquaporin gene expression and also, because of anatomical traits that result in less chloroplast surface exposure to intercellular space per unit leaf area.62 Stomatal closure, however, not only reduces water loss through transpiration but also reduces CO2 and nutrient uptake and thereby, altering photosynthesis.63 Dry-climate plants have evolved xeromorphic characteristics to minimize transpiration during water stress. Under water-stressed conditions, leaf shedding and reductions in leaf production, leaf size, and branching are additional ways to reduce transpiration loss. Another adaptation to counter water stress is sclerophylly, where plants develop hard leaves that will not suffer permanent damage from wilting and can be restored to full functionality once normal conditions return.64
Photosynthesis
Water stress inhibits photosynthesis by reducing leaf area and the photosynthetic rate per unit leaf area.64 The primary causes of reduced photosynthetic rate are stomatal closure or metabolic impairment (Figure 2).65 During photosynthesis, CO2 and H2O in chloroplasts of plant cells manufacture sugars and O2 as a by-product in the presence of light. Chlorophyll is a crucial part of chloroplasts, which are necessary for photosynthesis.66,67 The photosynthetic system and its pigments including chlorophyll a, b, and carotenoids are profoundly affected by drought stress (Figure 2).68 Plant starch synthesis is severely impacted by water deficit because it alters the Calvin cycle and enzyme activity.69 Chlorophyll pigments are vital for photosynthesis and are affected by water stress, affecting the opening and closing of stomata in plant leaves.70 In many plants, water stress increased oxidative stress, degradation of chlorophyll pigment, photo-oxidation, and chlorophyll concentration.71 Water sensitivity was mostly associated with a decrease in stomatal conductance, which lowered the transportation of CO2 to lower net photosynthesis.72,73 The major factors responsible for slowing down photosynthesis might be stomatal closure (reduced stomatal CO2 fixation) (Figure 2), non-stomatal (decreased photosynthesis activity in mesophyll tissues), or both.74 Water stress is a harmful abiotic stress that hinders photosynthesis by damaging the photosystems II (PS-II) because of their high susceptibility to external stimuli.75,76 Many studies on crops have demonstrated that carotenoids are less sensitive to water stress than chlorophyll. It was shown that plants under water stress produce more xanthophyll pigments such as zeaxanthin and antheraxanthin. In Japanese mint (Mentha canadensis),77 showed that severe water stress reduced the leaf solute potential, chlorophyll, and carotenoid contents with a greater loss of chlorophyll b than chlorophyll a. Besides, water stress instigates quick decline in photosynthesis that culminated in the reduction in the rate of ribulose-1,5-bisphosphate regeneration, the maximal rate of ribulose-1,5-carboxylate, NADP-malic enzyme, phosphoenolpyruvate carboxylase, Rubisco, fructose-1,6-bisphosphatase, and orthophosphate-Di kinase pyruvate.78
Cell size and respiration
Plant growth is governed by the processes of cell division, cell expansion, and cell differentiation, as well as genetic, ecological, and physio-morphological mechanisms.79 Cell development is one of the physiological processes that is markedly affected by water stress when turgor pressure falls.68 Water stress in higher plants can impede cell elongation by obstructing the flow of water from the xylem to adjacent elongating cells, ultimately leading to the mortality of the plant.80 An increase in the thickness of the cytoplasm suggests that higher levels of dissolved substances can harm the functioning of enzymes. The respiration rate is lowered in a broad range of plant parts during water stress including leaves, shoots, and the entire plant. On the other hand, a study conducted by Wahab et al.57 showed that under water stress conditions, plant respiration rates remain unaltered or even elevated.
Leaf relative water content
The leaf relative water content (RWC) is a crucial regulator of physiological processes in plants. Leaf RWC is used to estimate the water condition in plants. It represents the equilibrium between the amount of water supplied to the leaf tissue and the rate at which water is lost through transpiration.81 The decline in RWC is the primary symptom of water stress (Figure 2).82 The rate of growth and transpiration of leaf tissue are both substantially correlated with the relative water content of leaves.83 Lower RWC reduces the leaf water potential which enables the stomata to shrink. The main mechanism controlling leaf temperature is transpiration; decreasing the transpiration rate in leaves and raising leaf temperature are achieved through increasing stomatal resistance (Figure 2).4 Under stress regimes, a decrease in relative water content resulted in a reduction in water content and osmotic potential in plants. A reduced soil water potential during water stress hindered crop development and lowered plant osmotic potential, which results in inadequate nutrient uptake.84
Accumulating biochemical compounds including proline, protein, sucrose, and glycine betaine (GB) improve crop productivity by minimizing ROS-generated oxidative stress.58 Likewise, water stress affects physiological systems such as cellular respiration, photosynthetic rate, mineral nutrition, enzymatic activity, and cellular redox (oxidation/reduction) homeostasis. Consequently, biochemicals such as membrane lipo-proteins, DNA, and cellular protein are degraded in conditions of water deprivation.85 Accumulation of compatible solutes also known as osmo-protectants and organic acids in the cytoplasm was noticed in response to water stress. These solutes aid in scavenging ROS, improve water potential, and safeguarding biological molecules from lipid peroxidation.57 Under water stress, plant cells aggregate soluble substances and thicken their cytoplasm. In a comparable pattern, noncyclic electron transport was diminished to meet the requirements of decreased NADPH, ATP and ROS synthesis (Figure 2).57
Reactive oxygen species
Water-stressed plants have an increase in the formation of ROS, which occurs along their regular metabolic processes such as aerobic metabolism.86 The response of plants to water stress, either through photosynthesis or other means, results in oxidative damage of proteins, lipids, and nucleic acids. Since plants are sessile, they have evolved a plethora of techniques to assist them in adapting or surviving water stress.87 Enhanced ROS generation is inevitable in water stress conditions. However, phytotoxic amounts of ROS are perilous,88 and can cause cellular damage and even cell death.89 However, trace levels of ROS function as an essential signaling molecule that stimulates multiple stress-response pathways that commences crosstalk among them. The antioxidant system comprised of enzymes that produces and scavenges ROS and also, regulates the redox state of cells by reducing intracellular ROS levels. 90
Total phenolic content and osmotic adjustment
Phenolics are the most abundant and extensively dispersed phytochemical class in plants.91 Plants produce many of these phenolic compounds that are involved in their responses to biotic and abiotic environments. Previous studies indicated a 100% increase in phenolic content under water stress conditions.92 For instance, drought-stressed tomatoes (Solanum lycopersicum) had more total phenolic than well-watered plants.57 An osmotic adjustment occurs when the water potential in dividing cells is reduced, which maintains turgor pressure by accumulating solutes.93 Osmotic adjustment has been linked to sustaining stomatal conductance, photosynthesis, leaf water volume, and growth under water stress (Figure 2).94 The recognized major solutes that are build up in response to water stress are inorganic cations, organic acids, carbohydrates, and free amino acids. Studies demonstrated that the build-up of suitable solutes such as proline and glycine betaine protects plants against the damaging effects of water stress not only through osmotic adjustment but also by detoxifying ROS, maintaining membrane integrity, and stabilizing enzymes or proteins.64 It has been revealed that some enzymes including ornithine-aminotransferase, pyrroline-5-carboxylate reductase, and betaine aldehyde dehydrogenase play important roles in osmotic adjustment. However, several plant sugars such as sucrose, trehalose, glucose, and fructose are primary osmolytes that are important in osmotic adjustment.57
Flooding stress
When soil is flooded, plants are most severely harmed by lack of oxygen, which negatively affects mitochondrial respiration.95 Under severe flooding conditions, the impairment of oxidative phosphorylation of mitochondrial respiration occurs which eventually reduces the production of respiratory adenosine triphosphate. To make more ATP and combat the energy crisis under water stress conditions, plants increase glycolytic flow, which causes sugar stores to be depleted more quickly.96 Plants must produce enough ATP under these stressful conditions to maintain cellular processes and must replenish oxidised NAD+ to maintain the glycolytic flux. Pyruvate collected from glycolysis can be directed through fermentation pathways to rebuild the pool of NAD+ required for glycolysis under flooding conditions.97
Plants can use pyruvate as a substrate for two different fermentation processes: ethanol fermentation and lactate fermentation. Pyruvate decarboxylase (PDC) converts pyruvate to acetaldehyde during the fermentation of ethanol, and alcohol dehydrogenase (ADH) reduces this to ethanol while concurrently oxidising NADH to NAD+.98 To meet the energy requirements of cellular processes, ethanol fermentation must operate at greater rates due to the significantly lower energy production of ethanol fermentation (2mol ATP per mol glucose eaten) compared to mitochondrial respiration (36–38 mol ATP per mol glucose ingested).99 In trees that are tolerant to flooding, a substantial amount of ethanol created by ethanol fermentation in flooded roots may be transported to leaves by the transpiration stream.100-102 ADH and aldehyde dehydrogenase in the leaves oxidise it there, turning it into acetaldehyde and acetate in succession.103 Acetate-activating enzymes transform acetate into acetyl-CoA and re-enter central metabolism, recovering carbon that would otherwise be squandered as ethanol in hypoxic tissues.104 Lactate dehydrogenase transforms pyruvate to lactate during lactate fermentation, which also results in the oxidation of NADH.105 As lactate is a weak acid, its build-up may result in cellular acidification, which may induce enzyme deactivation and cause cell damage.97
In parallel to the modification in carbon metabolism via ethanol and lactate fermentation, oxygen deprivation has a substantial effect on nitrogen metabolism in plant cells.106 One of the amino acids that accumulate most dramatically when there is a lack of oxygen is alanine.107 Alanine aminotransferase (AlaAT), which favours the conversion of pyruvate and glutamate to alanine and 2-oxoglutarate under flooded conditions, is the main pathway for anaerobic deposition of alanine.108 In flooded conditions, freshly generated glutamate may be produced by NADH-dependent glutamate synthase (NADH-GOGAT) through the reductive amination of 2-oxoglutarate.109 Moreover, the enhanced NADH-GOGAT activity regenerates the NAD+ required to keep the glycolytic flux functioning in the event of low oxygen levels.106 Another way for anaerobic organisms to accumulate alanine is by a process called a GABA shunt, in which glutamate-derived GABA is changed into succinic semialdehyde while also changing pyruvate into alanine.110 It has been suggested that the accumulation of alanine and GABA under oxygen deprivation serves as an adaptive mechanism to protect the carbon that would otherwise be lost during the fermentation of ethanol and also, conserve ATP that would otherwise be used for assimilation of glutamine and asparagine by ATP-consuming enzymes. Many other amino acids including aspartate, glutamate and tyrosine have been found to change in a variety of species when they are under flooding stress.97 Moreover, during waterlogging, the levels of photorespiratory intermediates such as serine, glycine, glycolate, and glycerate elevated in the roots of Medicago truncatula, indicating a higher rate of photorespiration, possibly because of the reduced stomatal conductance.111
During stress, the tricarboxylic acid (TCA) cycle functions non-cyclically (Figure 3).109 The production of 2-oxoglutarate, which can enter mitochondria to form succinate via 2-oxoglutarate dehydrogenase and succinate CoA ligase occurs simultaneously with the anaerobic accumulation of alanine. This extra ATP production helps to make up for the lack of energy caused by oxygen restriction.97 Malate dehydrogenase converts oxaloacetate to malate, which is used by the mitochondria to metabolise 2-oxoglutarate.112 It is typical for succinate to accumulate during hypoxic conditions brought following flooding since succinate dehydrogenase (SDH), a TCA cycle enzyme, requires oxygen to function.109 In various plant species, changes in other TCA cycle intermediates like citrate, malate, and fumarate take place under flooding stress (Figure 3).113
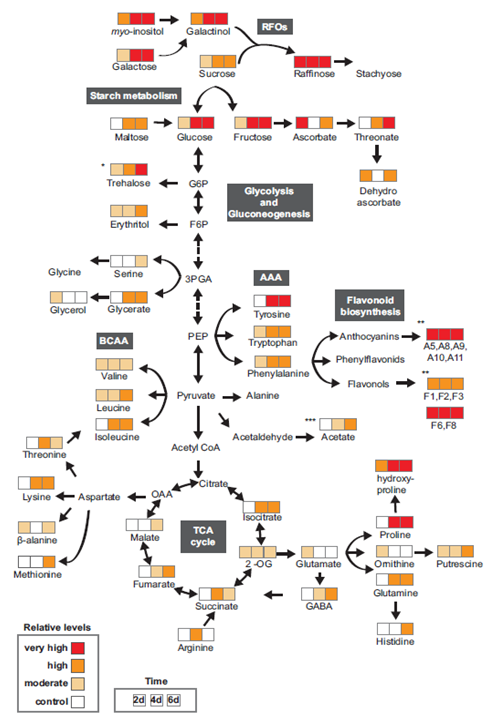
Figure 3 Metabolic response of wild-type Arabidopsis plants to water stress. The data for this images is extracted from Fàbregas et al. (2018), Pires et al. (2016), Kim et al. (2017) and Nakabayashi et al. (2014). Boxes show the 2, 4, and 6 day water stress times (d). In comparison to 0 days of water stress, colours represent the relative accumulation levels of each metabolite: Red, extremely high (>4); orange, high (2-4), pink, moderate (1-2), and white, no appreciable changes from control. RFOs are raffinose family oligosaccharides. AAA stands for aromatic amino acids. BCAA stands for branched-chain amino acids.
Drought stress
The restricted water supply in drought-stressed plants inhibits photosynthesis and impairs plant growth and development (Figure 2). Due to stomatal closure and higher mesophyll diffusional resistance, less CO2 is transported from the environment to the sites of carboxylation within chloroplasts during drought, which results in lower net CO2 uptake. According to some research, photosynthesis is restricted more directly by CO2 diffusional resistances under water deficits than by metabolic constraints.114 Water-stressed leaves with reduced photosynthesis are exposed to surplus energy because photosynthesis is the primary sink for photosynthetic electrons, which generates ROS that can impede ATP synthesis.115 There is an indication that under drought, ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco) activity declines, possibly connected to a reduction in ATP and Rubisco activase activity.56 Photorespiratory flux in the leaves of C3 plants under drought improves substantially as CO2 availability declines, causing electron sinks to contribute and leading to high rates of H2O2 production.97 An imbalance between the supply and demand of ATP or NADPH could be the underlying cause of the alterations in the size of metabolic pools driven by drought stress.116
One of the primary ways used by plants to maintain positive turgor pressure in water-limited situations is an osmotic adjustment, which involves the aggregation of solutes.117 Chemically, the osmolytes that build up during drought stress include soluble sugars (such as glucose, fructose, sucrose, and trehalose), raffinose family oligosaccharides (RFOs), amino acids (such as proline and GABA), quaternary ammonium compounds (such as glycine betaine), and polyamines (e.g., putrescine and spermidine).118 The accumulation of many of these osmolytes are also influenced by other abiotic factors like salinity, temperature, and flooding.119 Soluble sugars have an important role in osmoregulation, as well as in maintaining a balance between the supply and consumption of carbon and energy in plants under water stress. They also act as signalling molecules that govern a multitude of physiological and developmental processes.97
Many studies have shown that in response to drought stress, sugars such as glucose and fructose often build faster and more quickly than many other metabolites.118 In response to stress, the buildup of amino acids such as proline and GABA occurs later than that of carbohydrates. Glutamate dehydrogenase (GDH), which reversibly catalyses the synthesis of glutamate by the amination of 2-oxoglutarate generated by the TCA cycle could serve as an alternate source of ammonium.120 When plants are ATP-limited under drought, the increased GDH activity and the corresponding rise in proline levels imply that the GDH may become essential for ammonium absorption.97 Under water stress, several plant species exhibit an increase in branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine.121
Among the plethora of environmental stresses, water stress leads to significant oxidative stress which causes a substantial overlap in plant physiological and molecular responses (Figure 2).122 Consequently, measuring the amounts of specific metabolites can help determine the physiological status of a plant and the activities that are most probable to be affected by oxidative stress.123 Many soluble sugars including sucrose, fructose, raffinose, sorbitol, mannitol, and fructans have been demonstrated to have antioxidant capabilities in plant tissues in vitro, and in certain model systems, including fish oil-in-water emulsion.
Sugar and sugar derivatives
Independent investigations have identified substantial changes in metabolic pathways under oxidative stress including down-regulation of glycolysis and the TCA cycle and activation of the oxidative pentose phosphate pathway (Figure 3).124 The redirection of glycolytic carbon flow into the oxidative pentose phosphate pathway is primarily credited for the changes in the sugar profile and changes in the quantities of sugar phosphates and soluble carbohydrates with antioxidant properties (Figure 3).125 In Arabidopsis cell culture treated with H2O2, a rapid accumulation of sucrose and fructose followed by a rise in glucose level was observed.126 Further validation for the reprogramming of sugar metabolism came from the reduced starch accumulation in transgenic Arabidopsis plants that occurred naturally under oxidative stress because of the overexpression of the glycolate oxidase gene.127 Multiple studies have consistently found that the stress caused by drought and flooding situations accelerates the process of glycolysis. This is a method used to provide energy for activating stress defence mechanisms and adapting to stressful conditions.128 Furthermore, water stress triggers "energy crises" by impairing the oxidative phosphorylation process in the mitochondrion, leading to a significant decrease in ATP generation.95,96 Scientists have demonstrated that the buildup of sugar is vital in determining how plants distribute their carbon resources and promote development.129 In order to endure the "energy crises" caused by stressful conditions, resilient plants enhance their glycolytic influx by collecting additional glucose.128 This allows them to create an adequate amount of ATP through glycolysis, which is essential for sustaining fundamental cellular processes and replenishing NAD+ to sustain the flow of glycolysis.95
Water stress leads to excess concentrations of ROS, which are accompanied by an accumulation of oligosaccharides as α-galactosyl extensions of sucrose.130,131 These oligosaccharides had a higher degree of polymerization. Raffinose, one of these oligosaccharides, was identified in many plants while stachyose and verbascose, two with a higher degree of polymerization were only found in a few species and operate as osmo-protectants and stabilisers of cellular membranes.132 Several lines of evidence suggested that these metabolites may enhance plants adaptation to oxidative stress.131,133,134
Metabolites of tricarboxylic acid cycle
Being substrates for numerous biosynthetic pathways, many TCA cycle intermediates exhibit a highly dynamic character in their levels, which indicates the processes of metabolite creation and consumption (Figure 3). Yet, it has been demonstrated that the quantities of TCA cycle metabolites rise when respiration is increased, and under oxidative stress, plant cells have displayed a characteristic drop in the pool of TCA cycle metabolites.135 It appears that modifying the activity of TCA cycle enzymes has a significant effect on how the metabolic profile changes during oxidative stress. Several TCA cycle enzymes including aconitase, pyruvate-dehydrogenase and 2-oxoglutarate-dehydrogenase are vulnerable to oxidative stress.136,137 Heterotrophic Arabidopsis and rice (Oryza sativa) cultured cells and roots demonstrated a decrease in TCA cycle metabolites under oxidative stress.126
Contrastingly, numerous other studies have demonstrated that various plants undergo alterations in malate, citrate, α-ketoglutaric acid, and fumarate levels in response to flood stress.112,138,139 The presence of succinate increases significantly under conditions of hypoxia caused by flooding, as succinate dehydrogenase relies on the availability of oxygen.97,140 Furthermore, both drought and flood stresses induce the accumulation of citrate in plants.139 Furthermore, citrate plays a role not only in amino acid metabolism but also functions as an antioxidant and intermediate in respiratory metabolisms to produce energy for defence pathways in stress adaptation mechanisms.141 Moreover, α-ketoglutaric acid has a significant function in the process of respiration and the assimilation of nitrogen for the production of proline, glutamate, glutamine, and arginine. These amino acids play a role in controlling osmotic potential and serve as osmolytes to preserve protein integrity and facilitate water stress tolerance in plants.128
A summarization of individual studies revealed that approximately 55 metabolites are affected by drought, and 46 metabolites are affected by flooding (Figure 3).97 Interestingly, 23 metabolites showed common stress responses to various abiotic stresses such as cold, heat, drought, flooding and salinity. Because photosynthesis and photorespiration are extremely sensitive to environmental changes, several steps in the Calvin-Benson cycle (CBC) and photorespiratory pathway respond to water stress. Induced stress in plants results in oxidative damage, which changes the core metabolism of the plant. The development of protective compounds such as appropriate solutes or osmolytes to shield plants from oxidative damage, and the prevention of ROS formation are common themes for metabolic changes in response to oxidative damage.122,142 Compatibility-promoting solutes such as sucrose, trehalose, raffinose, mannitol, sorbitol, inositol, and proline proliferate in response to water stress (Figure 3). These compounds have high water solubility, high concentrations of non-toxicity, and low water activities that reduce protein-solvent interactions.143
The shikimate pathway is activated by several abiotic stresses, which results in an accumulation of aromatic amino acids like tyrosine, phenylalanine, and tryptophan. These aromatic amino acids serve as the building blocks for the manufacture of antioxidant phytoalexins, alkaloids, and flavonoids.144 Water stress can also trigger the production of sulfur-containing metabolites such as glutathione, methionine, and cysteine (Figure 3). These metabolites play crucial functions in the antioxidant systems of plants.145,146 Oxidative damage brought on by water stress may increase sugar phosphates linked to glycolysis and the oxidative pentose phosphate pathway (OPPP), as well as a decline in TCA cycle intermediates and amino acids generated from the TCA cycle.147
Under water stress, GABA is rapidly deposited.148 The primary role of GABA are signalling molecule, osmo-regulator, and cytosolic pH regulator.149 The accumulation of GABA in response to water stress stimuli often shares patterns with that of the BCAAs (valine, leucine, and isoleucine) and other amino acids that share their synthesis pathways (lysine, threonine, and methionine).150 The healthy growth of plants depends on BCAAs, which can also serve as suitable solutes, alternative electron donors for the mitochondrial electron transport chain, and substrates for secondary metabolites that are protective such as cyanogenic glycosides, glucosinolate, and acyl-sugars.151
Variations in ethanol, acetaldehyde, and acetate, which are intermediates in the fermentation of ethanol are more specifically related to flooding stress. The ability of a plant to withstand flooding may be related to its ability to recover the carbon that would otherwise be lost as ethanol by turning it into acetate and then acetyl-CoA to keep the flooded plant carbon metabolism running uniterrupted.103 Acetate is a key metabolite that was recently found to be involved in a distinct drought-survival pathway. Plant drought tolerance is enhanced by the metabolic transition from glycolysis to acetate production via pyruvate decarboxylase and aldehyde dehydrogenase.152 Moreover, engineering boosted the expression of the pathway for acetate production, and exogenous acetic acid administration improved plant life under drought.153,154
The economic importance of medicinal and aromatic plants is enormous because of the ongoing and expanding demand for their products in the domestic and international markets. The quality of medicinal and aromatic plants is contingent on the composition of plant secondary metabolites (PSMs), which are themselves influenced by environmental conditions such as water stress.155,156 Studies have shown that metabolite composition under stress depends on plant species, the compound being studied and the cultivation conditions. Many studies have noticed alterations in the composition of essential oil extracted from aromatic plants.157 Japanese mint plant fresh and dry weights, nutritional content, and essential oil output were all remarkedly reduced as a result of water stress.77 For instance, linalool and methyl chavicol concentrations in sweet basil essential oil increased as drought stress intensified.157 In comparison to the control treatment, the essential oil percentage of Thymus carmanicus rose by 12.5% and 44.9% under mild and severe water deficiencies, respectively.158 This observation can be ascribed to an increase in essential oil accumulation through a higher density of oil glands brought on by a diminution in leaf area under drought. However, in moderate and severe water deficiencies, the oil output was reduced by 43 and 44%, respectively. A study on Thymus spp. Revealed that water treatment at 67% field capacity resulted in a high essential oil yield compared to low yield at 33% field capacity. It is critical to remember that the amount of essential oil does not always increase in proportion to the severity of water stress because under more extreme conditions, the plant uses its assimilates to produce osmotic regulator compounds like proline and glycine-betaine as well as sugar compounds like sucrose, fructose, and fructan to provide the conditions it requires to thrive.159 Research conducted on basil highlighted that in response to water stress, the essential oil content per dry mass of the plant only slightly increased. Water supply changed the oil's quantitative makeup, lowering the amount of linalool from about 44% to 60%. Some trace amounts of ß-myrcene and 2-octanone were absent in plants under regular watering but were found in water-stressed plants.160 The amount of essential oil that aromatic plants contain depends on a variety of factors including species, environmental circumstances, and the degree and duration of water stress.
Water stress is a common threat, particularly in dry and semi-dry regions, and can impact all aspects of plant growth, development, and metabolism. The impact of water stress on plants is intricate due to its multifaceted nature. Global warming-related climate shifts are causing uncommon weather patterns around the world, most frequently in the form of drought and flooding. Plants have evolved intrinsic defenses against water stress through the process of evolution. The ability of all plants to respond to water stress varies. Even in highly tolerant plant species, tolerance is achieved by alterations in molecular and physiochemical processes that enable plants to adjust morphologically to water stress. However, they must pay a price for this tolerance in the form of decreased photosynthesis, which frequently results in lower biomass yields due to the cautious water management strategy used by plants. The genetic mechanisms that assist plants in producing enzymes, proteins, and chemicals suitable in various ways to fight water challenges are the reason for plant adaption to stress conditions. Plants experiencing water stress typically exhibit increased concentrations of natural compounds. This rise may be attributed to a decrease in biomass production with a substantial increase in the overall content of secondary metabolites. Many biotechnological interventions were developed to improve water stress adaptability of some high-value plants based on the understanding of the cause and effect of water stress on plants as well as the understanding of the responses plants make in different ways to become tolerant to such stress. But there are still a lot of gaps in our understanding of the causes and effects of water stress in plants, so we need to step up our efforts to gain a better understanding of the problem.
None.
The authors declare that there is no conflicts of interest.

©2023 Chada, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.