eISSN: 2576-4462


Heavy metals are dangerous pollutants of water and soil coming, in first place, from anthropogenic activity. Lead (Pb) can be accumulated in soil surface and it is easily take by plants inducing many symptoms of toxicity. Several research suggests that Pb toxicity leads to the induction of key enzymes of antioxidant defense system in tomato plants. This mini review will show the influence of Pb in some enzymes of tomato oxidative system focusing in the analysis of protein content and the enzymes glutathione reductase and superoxide dismutase in different parts of the plant (leaves and stem), some genes related to the oxidative stress of tomato were also study.
Keywords: Glutathione reductase, heavy metals, isoenzymes, lead, molecular mechanism, Solanum lycopersicum, superoxide dismutase
The accumulation of heavy metals in soils is mainly due to anthropogenic activity. Heavy metals are directly related to the risks of soil contamination, plant toxicity and the negative effects on the quality of natural resources and the environment, dangers dependent on various aspects such as the specific toxicity of the metal, bioaccumulation, persistence and non-biodegradability.1 In soil, the greatest danger lies in its accumulation by plants and transfer to animals, including humans,2 also these metals are not biodegradables. An example of heavy metals are: aluminum (Al), barium (Ba), beryllium (Be), cobalt (Co), copper (Cu), tin (Sn), iron (Fe), manganese (Mn), cadmium (Cd) , mercury (Hg), lead (Pb), arsenic (As), chromium (Cr), molybdenum (Mo), nickel (Ni), silver (Ag), selenium (Se), thallium (Tl), vanadium (Va) , gold (Au) and zinc (Zn).3
The absorption of Pb is a serious public health risk; causes retardation of mental and intellectual development of children, causes hypertension and diseases Cardiovascular in adults. Intoxication is due to the accidental ingestion of lead compounds or the ingestion by animals of forages or foods with lead, from environmentally contaminated areas.4 Heavy metals can be readily taken up by vegetable roots, and can be accumulated at high levels in the edible parts,5,6 tomato is one of these vegetables. For this reason is relevant to develop this study in leaves and stem of tomato plants where some enzymes and genes related to oxidative stress were study.
Growth reduction was observed in tomato plants of cv Micro-Tom under Pb-stress (Figure 1). Chlorosis and necrotic lesions appeared after 35 days of growth, indicating altered mineral nutrient absorption and photosynthesis.7 Tomato plants under Pb-stress for 35 days at the highest concentration of 10 mg kg-1 showed lost almost all the leaves, with reduction in growth length.7 Superoxide dismutase (SOD), Translationally Controlled Tumour Protein (TCTP) and Isoflavone Reductase (IFR)genes were studied to know their behavior at molecular and biochemical level in tomato plants cv. Micro-Tom under Pb stress. The three genes (SOD, TCTP and IFR) showed different expression profiles under the tested concentrations and depending on the collection phase (Figure 2). Ubiquitin was used as control gene due to its basal stable expression, as commonly used in different crops, with the ubiquitin-conjugating enzyme and elongation factor-1 regarded as the most stable based on their transcriptional profiles in Oryza sativa L. and Pennisetum ciliare.8,9
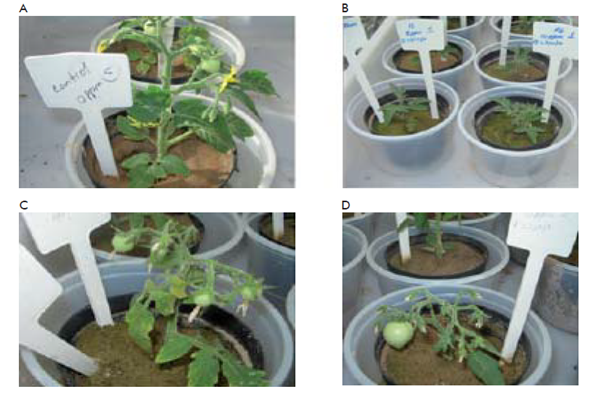
Figure 1 Plant growth at different lead soil content conditions. (A) Control without lead (PbAc2), respectively. (B) Growth at 5 and 10 mg/kg, left and right, respectively, ten days after transplantation. (C & D) Growth at 5 and 10 mg/kg of lead, 35 days after transplantation
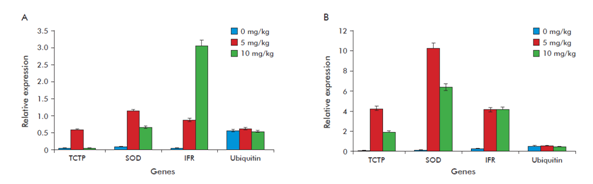
Figure 2 Relative expression of the transcriptionally-controlled tumor protein (TCTP), superoxide dismutase (SOD) and isoflavone reductase (IFR) in tomato plants grown on different lead (PbAc2) concentrations (0, 5 or 10 mg kg-1). (A) Phase germination-flowering. (B) Phase flowering-fructification. Ubiquitin was used as lead unrelated protein expression control.
SOD expression was lower in the first phase for both Pb treatments and higher in the second phase, specifically at 5 mg kg-1 of PbAc2. A possible explanation for second phase, as for other induced enzymes, may be caused by the possible temporal expression of SOD, this is the increased production of superoxide radicals due to the rise in ROS production by Pb toxicity. The higher expression of SOD at 5 mg kg-1 of PbAc2 in the mainly transitional. However, this response also may be a consequence of SOD-related transcriptional activity genes. So, in this study, SOD expression was higher at the lower heavy metal concentration.
A lower expression at 10 mg kg-1 of PbAc2 may suggest that higher concentrations could damage plants, which is suggested by the fact that the growth of plants at this concentration was affected. The expression of SOD at both Pb concentrations used in this research indicates that it may be involved in the antioxidative process under Pb induced stresses, since SOD is considered to be a crucial component in biological defense against oxidative stress.10
Biochemical characterization of S. lycopersicum cv. Micro-Tom
The biochemical analysis showed differences between SOD μmol min-1 per milligram of protein FW), GR (μmol min-1 per milligram of protein FW) and total proteins (Figure 3). During the experimental period, the content of SOD was higher at 10 mg kg-1 of PbAc2 with significant differences with respect to the control and 5 mg kg-1 of PbAc2. This is also corroborated by the fact that plant growth at this concentration was really affected. Plants exposed to Pb stress also show rapid and temporary drops in growth rate and activate antioxidant defense system by producing ROS, which alters gene expression and enzyme activity patterns of SOD. GR content was higher also at 10 mg kg-1 of PbAc2, with significant differences with respect to the other two treatments. This enzyme is part of the defenses system against oxidative stress. GRs are indispensable components of ascorbate-glutathione pathway, required to scavenge H2O2 produced mainly in chloroplasts and other cell organelles and to maintain the redox state of the cell.11
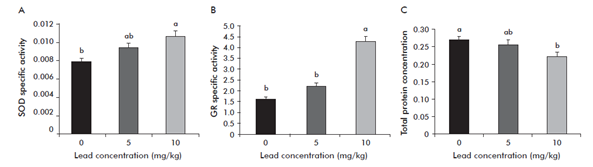
Figure 3 Effect of lead (PbAc2) concentrations on protein expression in tomato plants. (A) Superoxide dismutase (SOD) (μmol min-1 per milligram of protein). (B) Glutathione reductase (GR) (μmol min-1 per milligram of protein). (C) Total proteins of tomato plants.
This results show increased GR activity in Pb treated tomato plants, which suggests possible involvement of GR in regenerating GSH under Pb toxicity conditions to increase GSH/GSSG ratio and the total glutathione pool. Total proteins were higher in the absence of PbAc2 (control), which means there could be degraded with the other two concentrations used. Production of ROS takes place in cell under normal conditions, however adverse environmental conditions that interrupt cellular homeostasis could produce oxidative damage to proteins, DNA and to the lipids.12 This could explain why total proteins decreased at higher concentrations of Pb.
Molecular characterization of S. lycopersicum cv. Amalia
The seeds of tomato plants cv. Amalia were planted in trays containing a 1:1 (v/v) mixture of vermiculite supplemented with NPK (nitrogen, phosphorus and potassium) 10:10:10(g) and a commercial product (Plantmax HT Eucatex, Sao Paulo, Brazil ) at 2% weight: volume. The sowing was carried out in the period between September 2010 and December 2011 in a greenhouse located at the University of Sao Paulo/Higher School of Agriculture, Brazil. After the first pair of true leaves appeared, the seedlings were transplanted into 1 L Leonard containers (experimental unit) containing sand and three treatments were established with different concentration of Pb (0, 50 and 100 mg kg-1) and two part of the tomato plant cv. Amalia (leaves and stem).13
Biochemical characterization of S. lycopersicum cv. Amalia
In the treatments analyzed, no significant differences were found in the leaves in terms of total protein content, but in the stem the highest concentration (100 mg kg-1) was significantly higher than the control in 135.1% and that the treatment with 50 mg kg-1 of PbAc2 (136.59%), causing a significant decrease in the variable analyzed in both treatments (0 and 50 mg kg-1 of PbAc2) (Figure 4A & 4B). This increase in the content of total soluble proteins in the maximum concentration of PbAc2 used could be due to a saturation in the mechanisms of retention of Pb in the stem, and consequently the finite capacity of this organ to act as a reservoir of the metal,14 which continues to the leaves, where, although without significant differences, the protein content decreased at the maximum concentration tested. The activity of the GR in the presence of different concentrations of PbAc2 shows an increase in both parts of the analyzed plant being significant in the leaves (Figure 5A & 5B).
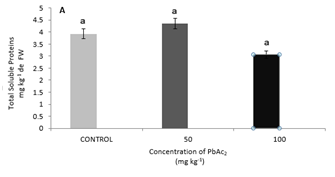
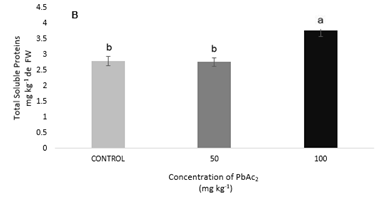
Figure 4 Total soluble proteins (mg g-1 of FW); (A) Leaves protein. (B) Stems protein of S. lycopersicum cv. Amalia Different letters indicate significant differences (p≤0.05).
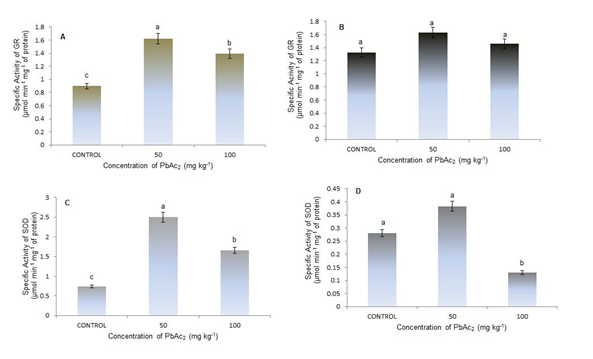
Figure 5 Enzyme activity (μmol / min / mg protein); (A) GR leaves. (B) GR stems. (C) SOD leaves. (D) SOD stems of S. lycopersicum cv. Amalia Different letters indicate significant differences (p≤0.05).
The activity of the GR in the leaves showed significant differences for the three treatments, with an increase when using 50 mg kg-1 of PbAc2 of 157.8% compared to the control, that is, with this concentration the activity of the enzyme responds to stress by Pb, possibly due to a requirement for glutathione in the reduced form as a substrate for incorporation into phytochelatins or the ascorbate-glutathione cycle to remove H2O2,15 however it is necessary to conduct future research on the synthesis of phytochelatins and other antioxidant systems including metabolites such as glutathione, ascorbate and amino acids which could be altered in responses to Pb.16
The activity of the SOD was significantly different in the leaves in all the treatments tested (Figure 5C) with the activity being higher at 50 mg kg-1 of PbAc2 in 342.47% compared to the control, followed by the treatment of 100 mg kg-1 which increased in a 227.40%. In tomato cv. Micro Tom Pérez et al.7 obtained the highest activity of the enzyme with 10 mg kg-1, the maximum concentration used taking into account that this cultivar in less than two months is fructifying, this demonstrates once again the detoxifying role of SOD. In the stems, significant differences were found between the maximum concentration of PbAc2 (100 mg kg-1) and the other treatments (Figure 5D). The lowest values of the enzyme were found (43.43% less than the control) in said treatment (100 mg kg-1). ); while with the lowest concentration of Pb there were no differences with the control.
In the leaves the behavior of both enzymes was similar increasing in the concentration of 50 mg kg-1 and decreasing in the maximum concentration used of the metal demonstrated that this concentration of tomato is toxic because it inhibits or reduces the activity of both enzymes. In the stem there were no significant differences in the activity of the GR but in the activity of the SOD significant differences were found being much lower at the maximum concentration tested, this behavior is due to both enzymes have different functions to combat oxidative stress, SOD is identified as an enzymatic protector against the peroxidation reactions that occur in the plant17 and GR is an indispensable component of the enzymatic ascorbate-glutathione pathway to eliminate H2O2 that is produced mainly in chloroplasts to maintain the redox state of the cell,18 indicating that the majority function of the GR is in the leaves.
We are very grateful to TWAS (Academy of Sciences for developing countries) and CNPq for providing the opportunity to carry out this research at the University of São Paulo / School of Agriculture "Luiz de Queiros" in Brazil.
Author declares that there is no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.