eISSN: 2469-2794


Research Article Volume 3 Issue 1
1Department of Nanoscience & Nanotechnology, Sri Sathya Sai Institute of Higher Learning, India
2Department of Physics and Astronomy and Clemson Nanomaterials Center and COMSET, Clemson University, USA
Correspondence: Venkataramaniah Kamisetti, Department of Nanoscience & Nanotechnology, Sri Sathya Sai Institute of Higher Learning, Prasanthinilayam, 515134, India
Received: July 25, 2016 | Published: November 4, 2016
Citation: Mulpur P, Kurdekar A, Podila R, et al. SPCE enabled acid-fast based onsite forensic sensors for the detection of Sperm cells/Semen. Forensic Res Criminol Int J. 2016;3(1):249-254. DOI: 10.15406/frcij.2016.03.00082
In this study we report the ultrasensitive detection of human semen/sperm cells enabled via a surface plasmon coupled emission (SPCE) platform that is of immense benefit from the forensic and criminology standpoint. The studies make use of the acid-fast property of sperm cells that endow the cells with the ability to retain fluorescent stains or dyes even upon acid-alcohol treatment, allowing their distinction and detection from other non-acid-fast cells that do not exhibit this property. SPCE studies on acid-fast stained semen and control samples were performed on the high-performance Ag-C60 substrates. In this regard, we were able to detect approximately 10 sperm cells/mm2; which highlights the ultra sensitivity of the Ag-C60 SPCE sensing platforms. While the acid-fast nature of sperm cells ensures specificity, SPCE assures sensitivity. We demonstrated the application of these substrates as onsite forensic sensors; enabling the rapid, specific, and ultrasensitive detection of acid-fast sperm cells or semen samples in an unambiguous manner. This is of immense relevance to forensic investigation and analysis of sexual assault cases. The positive identification of semen samples can enable forensics to perform DNA analyses. The detection of semen can also facilitate doctors to analyze the samples for suitable treatment modalities to be administered.
Keywords: surface plasmon coupled emission, ag-c60 substrates, sperm cells/semen, forensic sensors
GLAG, glass ag; SPCE, surface plasmon coupled emission; RhB, rhodamine b; RK, reverse kretschmann; (HCl-EtOH), hydrochloric acid-ethanol; AP, acid phosphatase; PSA, prostate specific antigen; STDs, sexually transmitted diseases; LWP, long wave pass; CW, continuous wave
The onsite detection of semen at a crime scene can enable forensic investigators to process collected samples and perform genetic analysis, for identifying the DNA fingerprint of the perpetrator. Semen, also known as seminal fluid, is a complex mixture of secretions from at least four male Urogenital glands. Based on the fact that semen contains various biomolecules, different sensing strategies have been developed to specifically identify particular biomarkers that are characteristic to semen samples. However, as it is with any sensing technique, while some tests enable the definitive and unambiguous identification of semen samples; others are deemed presumptive due to their lack of specificity. The use of microscopy to visually identify sperm cells is the ‘Gold Standard’ amongst all tests that allows unambiguous detection of semen.1 While microscopy allows the unambiguous identification of sperm cells and thus the semen sample, it is more of a laboratory method requiring infrastructure that occupies space and therefore is impractical for applying to onsite sensing scenarios. Another important drawback is that sperm cells undergo rapid degradation after ejaculation, as they cannot survive in dry and dehydrating conditions. Therefore, the elaborate process of sample preparation for microscopy may result in deterioration of the semen samples, which may already have been exposed to unfavorable conditions for quite a period of time at the crime scene.
Wood’s Lamp Test is another most popularly used onsite test for the detection of semen stains. In this test blue light or UV-light is used to visualize semen, which fluoresces due to the presence of molecules such as flavin and choline-conjugated proteins.2 Although the blue light based visual identification of semen samples is an extremely simplistic procedure; there are many other natural and synthetic molecules that fluoresce in a similar manner, making this test highly presumptive. Moreover, all semen stains do not fluoresce under such lights and the fluorescence can also be affected by conditions like heat, humidity, oxidizing agents and other microorganisms. Acid phosphatase test in which Acid phosphatase (AP) is an enzyme that is used as a biomarker for the detection of semen. AP, which is secreted by prostate glands, is a constituent of semen.3 On exposure to alpha-naphthyl acid phosphate and Brentamine Fast Blue stain, AP will produce a dark purple color in less than a minute (known as the Brentamine spot test). In this manner, the observation of a purple color indicates the presence of semen. However, the shade of the purple color in the AP test critically depends on the age of semen the stain and ambient conditions; where old stains may not produce the desired result. Importantly, there are other bodily fluids including vaginal secretions that contain detectable amounts of AP, thereby making this test also presumptive for the detection of semen.
Another test used for the detection of semen is the prostate specific antigen (PSA) test. PSA is secreted in high amounts by the prostate gland into semen and is usually detected by the ABA card or P30 test. While the PSA test is used quite commonly, it is also presumptive and not solely specific to semen. This is based on the fact that; small amounts of PSA can be found in fecal matter, sweat and even in female urine and breast milk.4 In the present work we demonstrate the application of Surface Plasmon Coupled Emission (SPCE)5-7 with Ag-C60 substrates as an onsite forensic sensor enabling for rapid, specific, and ultrasensitive detection of acid-fast sperm cells or semen samples. The ability of the Ag-C60 substrates to remarkably amplify fluorescence signals which we have reported before,8 is utilized in this study, to improve the sensitivity of fluorescence based detection of semen/sperm cells. This is of very significant to forensic investigations and analysis of sexual assault cases that are unfortunately prevalent worldwide. The positive identification of semen samples can enable forensics to perform DNA analyses, which may in turn aid the apprehension of criminal suspects. Furthermore, the detection of semen can also facilitate doctors to analyze the samples, to ascertain the risk of the victim being subject to unplanned pregnancies or contracting any sexually transmitted diseases (STDs) upon which, suitable treatment modalities can be administered.
SPCE enabled acid-fast based specific detection of sperm cells/semen
Acid-fast staining protocol to identify the sperm cells: Acid-fastness is a property of a cell that is resistant to decolonization by acids and/or alcohols during the staining procedure. In light of this aspect, the acid-fast staining protocol takes advantage of this acid-fastness of certain organisms and aids in their specific detection from other non-acid-fast organisms.9 Owing to the presence of a hydrophobic waxy lipid, namely, mycolic acid in their cell walls; acid-fast organisms prevent water-soluble dyes from entering their cell walls. Only stains, dyes or other fluorophores that are present along with a hydrophobic system can penetrate the cell walls of acid-fast organisms. Such dyes are known as acid-fast dyes or stains. Rhodamine b (RhB) in combination with glycerol and phenol is a well-known and good acid fast dye. In the context of the present study, it is noteworthy that the head of the sperm cells is an acid-fast structure and exhibits the ability to retain certain dyes or stains even upon performing decolorization procedures as described above. As can be seen from the Figure 1, the morphology of sperm cells consists of the characteristic head and tail structures. The head of the sperm cell is an acid-fast structure which lends a very significant advantage to achieve the highly specific detection of sperm cells because; there are no other acid-fast structures present in any of the other bodily fluids, such as; blood, urine, or sweat. While the sensitivity of the conventionally used sensing strategies (mentioned previously), are drastically affected by environmental factors like; temperature, age, humidity and rate of degradation of the semen samples; the mycolic acid present in the cell walls is an extremely tough biomolecule that has excellent chemical stability. Considering the fact that the acid-fast based sensing approach is based on the specific affinity of the acid-fast stains to the physically and chemically robust mycolic acid molecules; we can achieve the sensitive and specific detection of the acid-fast sperm cells/semen samples, irrespective of any modifications to the prevalent environmental conditions.10
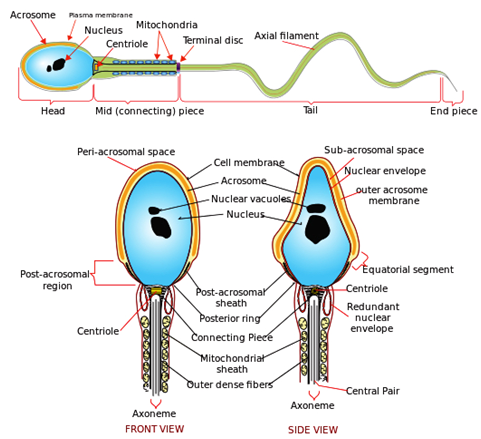
Figure 1 Diagram showing the detailed morphology and anatomy of sperm cells, which exhibit the distinct and characteristic head and tail structure. (Source: public domain).
Ag-C60 substrates as point-of-care sensors for detection of sperm cells/semen: In the present work, we used the Ag-C60 thin-film substrates, which remarkably enabled the realization of ultra sensitivity in detection of analyses. These high performance and robust substrates provide the best option as low-cost, point-of-care (POC) sensors, which can enable the rapid, sensitive and specific detection of sperm cells/semen samples from a forensic viewpoint.
Fabrication of GLAG thin-film architectures: Ag thin-films (~50 nm) were first deposited on pyrex glass microscope slides (75 x 25 mm) via physical vapor deposition to initially form the glass-Ag (GLAG) substrates. Subsequently, C60 spacer films of desired thickness (~10 nm) were directly sublimed onto the GLAG substrates to finally form the GLAG-C60 thin-film stacks.
Fixing and staining of sperm cells from semen samples
Fixing sperm cells and control samples: Semen samples from healthy subjects were procured from Sunshine Hospitals, Secunderabad, India. The concentrated stock semen sample, containing approximately ~2×107 spermatozoa/ml, was diluted in distilled water to achieve a 10% dilution. Subsequently, the diluted semen aqueous suspension was drop-casted and smeared across the GLAG-C60 substrates. After coating, the fixing or immobilization of the sperm cells was immediately performed, by gently heating the semen coated SPCE substrates using a heating mantle. Care was taken not to overheat the substrates, as it may result in the degradation and destruction of the sensitive sperm cells. It is noteworthy that in addition to semen, other body fluids like; blood, saliva and urine, are also often encountered at crime scenes involving sexual assault. In this regard, the unambiguous detection of blood, which is also markedly different in color to the other body fluids, is already well established in forensic science. On the other hand, considering that semen is an almost colorless or translucent fluid, which can appear similar to other colorless body fluids like saliva and urine at a sex crime scene; we also performed fixing, staining and SPCE studies on saliva and urine samples that were deemed as controls in this work. The control samples were also fixed onto the GLAG-C60 substrates in a manner similar to the semen/sperm samples.11
Acid-Fast Staining Protocol: A fluorophore solution of rhodamine b (RhB) was prepared in a mixture of water, phenol and glycerol in 5:2:15 ratios. A 0.5% hydrochloric acid-ethanol (HCl-EtOH) mixture was prepared for the acid-treatment/decolorizing process. The SPCE substrates coated with both acid-fast sperm cells and, the saliva and urine control samples, were initially heated to melt the mycolic acid layer of the acid-fast biological samples. The substrates were then flooded with the RhB solution that was allowed to stand for three minutes. Excess RhB solution was washed off using distilled water following which; the biological samples were subjected to acid-alcohol treatment by flooding the slide with HCl-EtOH mixture for one minute. Following acid treatment, the slides were again washed with distilled water and air-dried. A schematic depicting the acid-fast protocol for detecting sperm cells/semen samples is shown in Figure 2. In the present study we demonstrate the utility of SPCE substrates as a non-microscopic sensing platform and hence further conventional steps of the standard acid-fast protocol, consisting of elaborate counterstaining steps with KMnO4 and subsequent wash procedures are not required, and therefore were not performed.
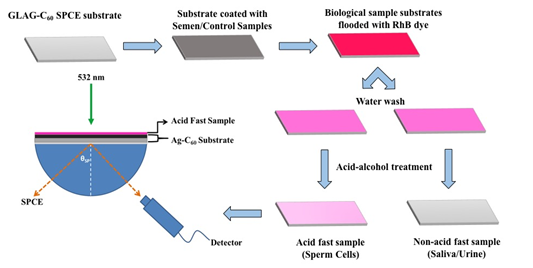
Figure 2 (a) A schematic depicting the use of GLAG-C60 SPCE substrates in the Reverse Kretschmann (RK) optical configuration, for the detection of sperm cells. In the optical setup: a laser beam directly excites the samples on the GLAG-C60 substrates and the generated SPCE signals are recorded by a fiber-optic detector. The schematic of the acid-fast staining protocol is also depicted in this figure where the semen and control samples are first coated onto the GLAG-substrates, heat-fixed, and subsequently covered with RhB. Acid-fact sperm cells are resistant to acid-alcohol decolorizing and thus fluoresce strongly with a strong plasmon coupled signal; while non-acid fast specimens namely; saliva and urine do not exhibit any SPCE signals.
Establishment of reverse kretschmann (RK) optical setup for SPCE studies: For performing measurements and conducting SPCE studies, we have used an optical table for constructing the optical setup in RK configuration. The setup comprises of the excitation source, optical components, detector and a computer monitor to display the recorded output SPCE signals. A schematic of the RK experimental setup is also depicted in Figure 2.
Excitation Source: In all our studies we have employed a p-polarized, 532 nm c.w. green laser, with a maximum operating power of 80 mW, as the incident excitation source (Shanghai Dream Lasers Technology Co. Ltd.). A green laser is suitable, as it provides appropriate energy for exciting the Rhodamine B (RhB) fluorophore molecules used in our studies, which have an absorption maximum at the wavelength λ=550 nm.
Optical components: For performing the SPCE measurements effectively in the RK optical configuration, we have utilized the following optical components in our setup. Power attenuator: For modulating and optimizing the output intensity of the incident excitation source, we have incorporated a power attenuator into our setup. The use of the attenuator facilitates suitable manual control for the prevention of detrimental photobleaching effects on fluorophores. Translational Mirror: It is important to study whether the observed SPCE profiles are displayed uniformly from the entire SPCE substrate. For this purpose, we used a translational mirror in our optical setup to effectively direct the incident laser beam onto every region of SPCE thin-film substrates, which can be controlled with the help of x-y plane control knobs. Rotary Sample Stage: The SPCE substrates used in our studies are fabricated on basal, glass microscope slides. These substrates were affixed onto a hemi-cylindrical prism that was placed on a calibrated rotary sample stage. The rotary stage is further equipped with a telescope through which an optical fiber based detector was fixed. This facilitates movement of the fiber in a complete range of observational angles (360 degrees), to record free space or SPCE signals in front of, and behind the SPCE substrate affixed to the prism, respectively. Long Wave Pass (LWP) Filter: A 550 nm LWP filter was incorporated as a fixed component that was attached to the telescope, such that it was always positioned between the detector and the SPCE substrate. The filter is used to cut-off any stray light emanating from the 532 nm laser, thereby allowing only SPCE signals (λ > 550 nm) to enter the detector.
Detector: For recording free space and SPCE signals, we used a portable Ocean Optics© fiber-optic spectrometer (model: USB4000 series). As mentioned, the fiber-optic cable of the spectrometer was fixed through a telescope, which is a component of the calibrated rotary stage. The fiber optic probe feeds recorded signals to the spectrometer that generates the signal spectrum which is viewed on a computer. In this manner, utilizing the various components and parts mentioned, we were able to construct a robust optical platform in the Reverse Kretschmann (RK) configuration that facilitated experimental measurements of SPCE signals in all our studies.
Measurement of signal enhancements in SPCE
Signal Enhancement: In SPCE studies, the factor of enhancement in fluorescence signal intensity is determined, by calculating the ratio between; experimentally measured SPCE signal intensity, with respect to the free space isotropic fluorescence signal intensity. The intensity is measured at a particular central emission wavelength, where maximal emission intensity is observed from fluorophores. This can be expressed as:
Signal Enhancement Factor=Intensity of SPCE SignalsIntensity of Free Space Signals
In the RK configuration, on irradiation with an incident source, the fluorophores are excited first. The isotropic fluorescence signal intensity can be recorded by positioning the fiber-optic detector on the frontal side of SPCE substrates; i.e. on the free space side of the sample. On the other hand, fluorescence-plasmon coupled emission signals arise through the prism, on the side opposite to free space. The SPCE signals can be recorded, by appropriately positioning the detector behind the prism, at an angle where maximal intensity of fluorescence emission signals is observed. The orientation of the detector for recording the free space and SPCE signals is depicted in the schematic shown in Figure 2. Based on the data obtained, the factor of SPCE based signal enhancements can be determined. For example; if the maximal SPCE signal intensity at the central emission wavelength is 1000 counts and free space intensity is 100 counts; the SPCE based signal enhancement factor is determined to be 10-fold. The spectral data is usually represented as an intensity plot with wavelength (nm) on the x-axis and the fluorescence signal intensity on the y-axis.
Fluorescence microscopy: We have also performed fluorescence microscopy on these samples, to validate that the SPCE signals indeed originate from the acid-fast structures. Fluorescence microscopy also enables us to assess the sensitivity of the SPCE platform that can be deduced by observing the number of cells being detected in the view scope area. The fluorescence microscope used in our studies is a Model BM-3000 FLT (LABEN Instruments); equipped with a Sony Cybershot© camera, DSC-HX1 series.
Imaging sperm cells via fluorescence microscopy: We performed fluorescence microscopy on the GLAG-C60 substrates that were coated with acid-fast sperm cells/semen and the control (saliva and urine) samples. Naturally, due to the absence of any acid-fast structures, absolutely no fluorescence was observed from the control coated substrates. The fluorescence microscope imaging of the semen coated substrates was performed in two operational modes of the instrument namely; white-light and the fluorescence configuration respectively. In the white-light mode; thousands of sperm cells were observed, spread uniformly over the entire substrate; which can be seen in Figure 3. On zooming into the substrate, we were able to observe individual sperm cells distinctly, where the characteristic head and tail morphology of spermatozoa was clearly distinguishable. A photograph captured in the white-light configuration, clearly showing at least 3 distinct sperm cells, is given in Figure 3. Having identified the sperm cells clearly in the white-light mode, we shifted to the fluorescence mode of operation, where the substrates were excited with a 532 nm source in the microscope. Remarkably, the spermatozoa were found to exhibit strong reddish-orange fluorescence, characteristic of the acid-fast stain RhB, which distinctly emanated only from the heads of the sperm cells; shown in Figure 3. The observation that only the heads of sperm cells distinctly displayed fluorescence stands as a testimony to the fact that; only the head of a sperm cell is an acid-fast structure. Furthermore, the selective and preferential staining of the sperm cells and not the control (saliva and urine) samples; substantiates the affinity of the acid-fast stain to the mycolic acid molecules present in the acid-fast sperm head structures.
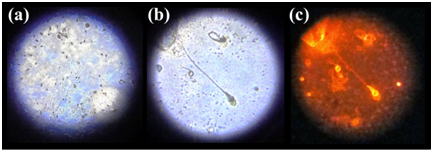
Figure 3 Photographs of sperm cells on Ag-C60 substrates under fluorescence microscope showing: (a) Numerous sperm cells under white light; (b) Zoomed in area of the substrate under white light showing sperm cells with distinct head and tail morphology; and (c) Photograph of zoomed area of sperm cells on green laser excitation, exhibiting orange fluorescence distinctly arising from the acid-fast heads that corresponds to the SPCE emission @580 nm.
SPCE based sensitive and specific detection of sperm cells/semen: SPCE studies on acid-fast stained semen and control samples, which were performed on the high-performance Ag-C60 substrates. On excitation with the 532 nm laser source, the SPCE signals arising from the semen and controls coated GLAG-C60 substrates were recorded; and shown below in Figure 4. As we can see from the Figure 5, the acid-fast based SPCE sensing platform enabled the highly sensitive and especially the specific detection of sperm cells/semen over the saliva and urine samples. In this regard, the RhB stained semen samples displayed strong emission @580 nm that corresponds to the distinct reddish-orange color of fluorescence observed in microscope imaging; (c.f. Figure 3). The SPCE signals were ~10-fold more intense, than the corresponding free space emission signals; thereby highlighting the high sensitivity of the SPCE platform.
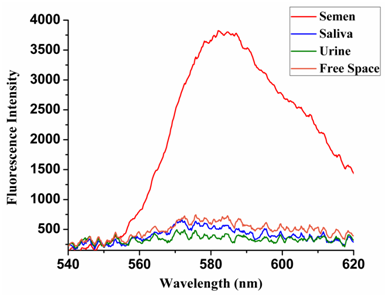
Figure 4 A fluorescence intensity plot showing the recorded SPCE signal intensities arising from GLAG-C60 substrates coated with acid-fast sperm cells (strong SPCE signals) versus; non-acid-fast saliva and urine control samples (negligible fluorescence).
Specificity of Semen Detection: From Figure 4, it can be seen clearly that strong SPCE signals were only recorded from the semen coated substrates, whereas the control saliva and urine coated substrates exhibited negligible/nil fluorescence. To reiterate, this specificity is attributed to the acid-fast nature of the head of sperm cells, which contain mycolic acid. Owing to this attribute, on acid-fast staining, many RhB dye molecules are retained within the cell membrane of the sperm head and thereby resist decolorization. As a result, on excitation the sperm heads fluoresce brightly. On the other hand, there are no acid-fast structures in either the urine or saliva samples and therefore on performing the post-staining decolorization treatment, all the RhB dye molecules are washed away, leaving no fluorophores behind. As a consequence, even on excitation, absolutely no fluorescence is observed from the non-acid-fast control samples. Therefore, it is the acid-fast nature of sperm cells that is responsible for lending a high degree of specificity to the SPCE based sensing strategy.
Sensitivity of semen detection: In order to experimentally substantiate the sensitivity of the Ag-C60 substrates, we performed microscopic evaluation to determine the number of sperm cells that can be detected in a unit mm2 area. In this regard, we were able to detect approximately 10 sperm cells/mm2; which yet again highlights the ultra sensitivity of the Ag-C60 SPCE sensing platforms. In this manner, we report the ability to enable the immediate and prompt detection of sperm cells/semen, even from low sample volumes and concentrations which is beneficial to forensic and criminology analyses.
None.
None.

©2016 Mulpur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.