eISSN: 2469-2794


Review Article Volume 4 Issue 2
Department of Pediatric Dentistry, Asan Memorial Dental College and Hospitals, India
Correspondence: Ekta Priya, Senior Lecturer, Department of Pediatric Dentistry, Asan Memorial Dental College and Hospitals, Oragadam Road, Keerapakam, Tamil Nadu, India, Tel 91 9566404435
Received: November 17, 2016 | Published: February 20, 2017
Citation: Priya E. Methods of skeletal age estimation used by forensic anthropologists in adults: a review. Forensic Res Criminol Int J. 2017;4(2):41-51. DOI: 10.15406/frcij.2017.04.00104
Reconstruction of biological profile of unknown individuals would be incomplete without age determination. Forensic anthropologists use skeletal indicators involved in processes of bone resorption, deposition and remodelling which are time-related to estimate age of the individual. Estimating age in adults remains a challenging task to the forensic anthropologists because of the complexity and individual variations seen in the aging process and the gamut of environmental factors influencing the same. Age provided by anthropologists is determined as age range rather than a specific age. It has been noticed that age range determined for younger individuals is narrower than for older individuals. This paper reviews most commonly used skeletal age estimation methods by forensic anthropologists.
Keywords: forensic, anthropology, skeletal age, age estimation
Loss of identity, in cases of both the living and the deceased poses a frequent problem for individuals and authorities. Reconstruction of biological profile of unknown individuals would be incomplete without age determination. Age determination can be performed based on developing dentition, growing skeleton or degenerative changes of the skeleton.1 For adults, age can be estimated using indicators involved in processes of bone resorption, deposition and remodelling.2 The evaluation of degenerative processes in adults is based on the normal wear and tear of the body over time. These processes are susceptible to human behaviour and various environmental factors.3 The process of remodelling is highly variable from one individual to other and the alterations produced are often subtle and difficult to interpret. Furthermore, it is likely that these degenerative changes will differ in their timing and manner among various populations.2 It has been reported that determination of age for younger individuals, is found to have a narrower age range; whereas for older individuals, the estimated age has a wider age range. This can be attributed to the fact that for younger individuals there are many biological changes occurring at regular intervals and rates. After the individual attains biological maturity, the number and rate of developmental changes are reduced.4 In adult age estimation, middle age is probably the most difficult to assess as during this period of life, transitions are highly varied due to hormonal and metabolic changes. However, the later years of age have their own share of complications and difficulties which include inevitably occurring higher rates of pathologic conditions and the effects of wear and tear in one’s life.3 Accurate age at death estimate is essential for the analysis of skeletal remains by the forensic anthropologists, especially in a medico legal settings.5 Therefore, it is essential to understand the accuracy of the existing skeletal age assessment methods and also if necessary, modify these existing methods to conduct a more precise and reliable age estimation for the various global populations. Various methods of skeletal age estimation have been reported in the literature. The most commonly used age estimation methods used by forensic anthropologists in adults are based on the study of,
Other skeletal parameters such as examining the clavicle, acetabular surface, proximal femur are often used as methods of age estimation in adults in conjunction with the conventional methods.
Age estimation based on pubic symphysis
Pubic symphysis refers to the surface at which the two innominates articulate anteriorly. Different standards were introduced based on the morphological changes seen in pubic symphyseal area for age estimation. Initially, aging charts were developed using the cadavers from different medical schools. These samples comprised of the older individuals and who may have been in an unhealthy condition. Age estimation using pubic symphysis gained its attention when there was a need to identify individuals killed in the Second World War. Coming from the military context, these individuals belonged to younger age group ranging from 17 years of age to 25 years of age, and majority of them being males. Later, age changes seen in female pubic symphysis were also studied. One of the complicating factors faced in females is that child birth causes irregular wear and tear at the pubic symphyseal area. Two implications were noted, first, it is not encouraged to use the male standards for age estimation of the female symphysis, and vice versa; and second, the irregularities on the female symphysis caused due to giving birth or not having given birth produced greater variability.4 It has been reported in the literature that the morphology of the face of the pubic symphysis varies with the age and is one of the most widely used parameters for the purpose of age estimation. Todd in 1920 proposed a ten phase method for analysing the pubic symphysis to estimate age.6 The ten phases of morphological changes occurring in pubic symphysis described by Todd are shown in Table 1 and illustrated in Figure 1.
Phase |
Age Range |
Description |
1 |
18-19 |
Symphyseal face rugged, traversed by horizontal ridges separated by well-marked grooves, |
2 |
20-21 |
Symphyseal surface still rugged. Horizontal grooves are becoming filled near their |
3 |
22-24 |
Symphyseal face shows progressive obliteration of ridge and furrow system. Commencing formation of a dorsal platform. |
4 |
25-26 |
Great increase of ventral beveled area. Corresponding diminution of ridge and furrow formation. |
5 |
27-30 |
Little change in symphyseal face and dorsal platform. Margin more clearly |
6 |
30-35 |
Increasing definition of extremities. Development and practical completion of ventral rampart. |
7 |
35-39 |
Face and ventral aspect change from granular to fine-grained or dense bone. |
8 |
40-45 |
Symphyseal face and ventral aspect of pubic bone generally smooth and inactive. |
9 |
45-49 |
Symphyseal face presents a more or less marked rim. Dorsal margin uniformly lipped; |
10 |
49+ |
Ventral margin eroded at a greater or lesser extent of its length, continuing |
Table 1 Phases of morphological changes seen in pubic symphysis described by Todd
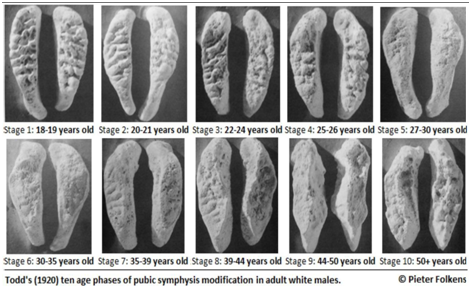
Figure 1 Ten phases of morphological changes occurring in pubic symphysis described by Todd.6
Todd’s method was revised several times by investigators in due course of time. These revisions were made with respect to the population under study and creating separate standards for females. One of the most widely used revised pubic symphysis method was introduced by Suchey and co-workers. In 1986 Suchey and co-workers revised Todd’s method of analysing morphological changes in pubic symphysis and outlined six staged method based on the sample of 739 males who were autopsied at the department of Chief Medical Examiner-Coroner, County of Los Angeles.7 In 1990 Suchey and Brooks added and 273 females to this sample and refined the method and demonstrated gender specific age ranges.8 The stages are described in Table 2 illustrated in Figure 2. Suchey and Brooks demonstrated that Todd’s standards overestimated age in older individuals. This revised method developed by Suchey and Brooks is one of the most commonly used age assessment method due to its large sample size and detailed description of the phases. Suchey and Brooks highlighted that their method can be appropriate to employ in a wide array of contexts as their sample of Los Angeles was derived from individuals belonging to diverse socioeconomic backgrounds throughout Asia, Europe, North America and South America. However, they suggested that whenever possible multiple age indicators should be used for more reliable and precise age estimation. They also warned that higher amount of variations was observed in the third and fourth phases of morphological changes.8
Phase |
Age Range |
Description |
1 |
15-23 |
Symphyseal face has a billowing surface composed of ridges and furrow |
2 |
19-34 |
Symphyseal face may still show ridge development. Lower and upper |
3 |
21-46 |
Symphyseal face shows lower extremity and ventral rampart in process of completion. |
4 |
23-57 |
Symphyseal face is generally fine grained, although remnants of ridge |
5 |
27-66 |
Slight depression of the face relative to a completed rim. Moderate lipping is |
6 |
34-86 |
Symphyseal face shows ongoing depression as rim erodes. |
Table 2 Description of phases of morphological changes in pubic symphysis by Suchey-Brooks
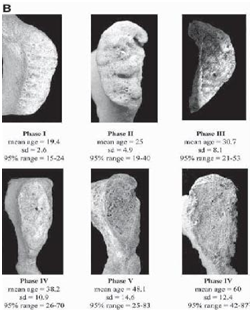
Figure 2 Phases of morphological changes seen in pubic symphysis proposed by Suchey-Brooks.12
Suchey and Brooks method was tested for its applicability in various populations. Bednarek et al.,9 tested this method among Polish males aged between 13 to 88 years. The results showed that the method can be applicable only for the group of younger males.9 Sarajlic et al.,10 tested its applicability among Bosnia and Herzegovina population and found that the standard deviation increased from phase to phase.10 However no conclusion was drawn, based on the fact that there occurred different span of age interval for each phase. When Djurić et al.,11 evaluated Suchey and Brooks method for aging skeletons in the Balkans, it showed an accuracy of 72.0% and 89.74% in females and males respectively.11 Lottering et al.,12 in 2013 conducted a study to test the applicability of Suchey and Brooks method on population of Queensland, Australia. It was reported that the bias ranged from -8.63 to 6.45 years for females and from -7.13 to 6.88 in males. Irrespective of the gender, bias was found to be highest in the age of 65-70 years. The results showed positive biases for younger individuals who were aged less than 55 years and negative biases for older individuals. The authors suggested that the Suchey and Brooks method should be applied with caution in medico-legal death investigations of skeletal remains in the region of Queensland, Australia and further investigations are warranted for reliable age estimation technique.12 In addition to revision of fourth rib method (discussed below), Hartnett revised the Suchey and Brooks’s method by adding a seventh phase for females and also altering the age ranges slightly for both males and females. This study was based on the modern sample derived from Maricopia County Forensic Science Centre in Phoenix, Arizona.13 These revised methods were tested by Merritt in 2014 in 1000 modern skeletons with known age of death. The results of the study proved that Hartnett’s revised pubic symphysis method performed significantly better in terms of accuracy and bias for the entire sample. When applied to separate age categories, Hartnett’s revised method demonstrated better reliability for older age individuals (>50 years) than younger.14 When compared to the fourth rib method, Hartnett’s revised method could show little improvement for old individuals.13
Age estimation based on auricular surface of the ilium
Auricular surface of the ilium provides an accurate estimate of age at death as the age changes seen are relatively well defined and regular. The advantages of auricular surface being used as a parameter for skeletal age assessment is that it has higher survival rate in archaeological populations and identifiable changes extend beyond the age of 50 when compared to pubic symphysis. However, interpreting the age changes in auricular surface is relatively more difficult when compared to pubic symphysis. This could be because of the complexity seen in aging process in the auricular surface and the “delayed epiphysis” stage which is found in the pubic symphysis. Few features which are regularly used while interpreting the morphological changes in auricular surface are described below.15
Porosity: Perforations seen in the region of subchondral bone of the auricular surface are referred to as porosities. Perforations which are smaller and just optically visible are called microporosities and perforations which are less regular, generally oval in shape and ranging from 1 to 10 mm in diameter are referred to as macroporosities.
Grain: The gross appearance of the surface which can be identified by unaided eye is referred to as grain of the surface. Heavily grained surface is comparable to fine sand paper.
Billowing: Presence or absence of transverse ridging on the auricular surface is referred to as billowing. The billows usually run transversely across upper and lower surfaces. It can vary from large regular surface to just visible fine grained ridges.
Density: Lovejoy et al.,15 described density as “the surface in which the subchondral bone appears compact, smooth and shows a marked absence of grain”
General surface changes with age
Lovejoy and co-workers qualitatively evaluated the absence or presence of the above mentioned features in 102 ilia from the Todd collection. It was interesting to know that there was sex differentials found in any of the features. The general changes occurring in these traits are described briefly below,15
Grain: Fine granular surface is an indicator of young age. Loss of granularity increases with increasing age. Complete loss of granularity can be seen at the age of 45-50 years. Subsequently, the subchondral bone becomes highly compact and no granulations are seen.
Macroporosity: Macroporosity if present is a general indicator of age. However, it should not be confused with the subchondral defects. Subchondral defects are present intermittently and are not systemic whereas macroporosities generally cover a large surface area. Until the age of 50, the presence of this feature is rare, after which it is noticed more frequently.
Billowing: Billowing is mostly present in the younger individuals. This feature declines after the age of 25 years and is replaced with striations.
Striations: Transverse striations characterize the individual surfaces in the fourth decade of the life. This feature is rarely found after the age of 50. The striations are more marked in the inferior surface. At the age of 35, the surface becomes granular and slightly striated without any presence of billows. Both billows and striations are found to rarely over the age of 40.
Apex: Until the age of 35, the edge of the auricular surface tends to be sharp and distinct. After this age due to arthritic lipping, the contour varies from broad triangular form to rim formation. Most of the arthritic changes can be appreciated and clearly interpreted at this region.
Retroauricular area: Young specimens show features like increase in porosities, general surface irregularities and presence of fine to large osteophytes at the retroauricular area. It is to be noted that this parameter of age estimation should be used in conjuncture with the other features of the auricular surface for more accurate determination of age.
Transverse organisation: Both billows and striations show the anteroposterior organisation of the surface. With the increase in age, there is a decline of these transverse organisation. This makes the surface appear more amorphous having no clear directional structure.
Phases of age changes at the auricular surface
As described by Lovejoy et al.,15 five basic phases can be seen during age transformation of the auricular surface. These are
Early post-epiphyseal phase: The epiphysis which appears to be irregular and plate-like, fuses to the sacral portion of the sacroiliac joint after the commencement of puberty. This generally lasts until the age of mid-20s.
Young adult phase: Loss of billowing and coarsening of granulation can be seen during this period. Not much changes are seen in the periauricular features. This period extends from mid-20s to the mid-30s or sometimes also seen in slightly older individuals.
Mid adult phase: This period extends from the mid-30s to the mid-40s where age changes in the retroauricular area are more marked. These periauricular activity prove to be useful in age estimation.
Early senescent phase: During this phase the age changes are seen in the features like grain, density, porosity and the apical condition of the auricular surface. The periauricular activity is further increased during this period. This phase lasts between the mid-40s and the mid-50s.
Breakdown: A progressive destruction is seen in the subchondral bone after the mid-50s. Increased porosities, irregularities and marked periauricluar activity can be noticed during this phase.
Chronological changes in auricular surface: Lovejoy et al.,15 observed over 250 preserved auricular surfaces from Libben population and systematic observation of around 500 specimens from Todd Collection. Using this sample the chronological stages of the auricular surface were recorded. The observed age changes in the auricular surface are summarized in Table 3.
S. No. |
Age Range |
Granularity |
Tranverse Organisation |
Retro Auricular Activity |
Apical Activity |
Porosity |
Billows |
Striations |
1 |
20-24 |
Fine granular texture |
Marked |
Not present |
Not present |
Not present |
Well defined |
Not present |
2 |
25-29 |
Slightly coarse |
Marked |
Not present |
Not present |
Not present |
Slight to moderate loss |
Present |
3 |
30-34 |
Coarser |
Reduced |
Not significantly present |
Not significant |
Small areas of microporosities |
Much reduced and replaced by striations |
Fine anddefinite |
4 |
35-39 |
Coarse and uniformly present |
Present but poorly defined |
Not significantly present |
Minimal changes |
Minimal changes |
Markedly reduced |
Reduced but may still be present under observation |
5 |
40-44 |
Coarse and partial densification seen |
Marked loss |
Slight to moderately present |
Slight changes |
Occasionally macroporosities seen |
Not present |
If present, very vague |
6 |
45-49 |
Significant loss |
Complete lost |
Moderately present |
Slight to moderate changes |
Lost to densification process surface irregularity seen |
Not present |
Not present |
7 |
50-60 |
Generally absent |
Not present |
Moderately to markedly present |
Invariable and may be marked |
Marked surface irregularity |
Not present |
Not present |
8 |
60+ |
Completely absent |
Not present |
Markedly present |
Marked |
Macroporosities may be present |
Not present |
Not present |
Table 3 Summary of chronological changes in the auricular surface
Test of accuracy and reliability
Lovejoy et al.,15 stated that correlation of the estimated age ranged from 0.55 to 0.75 at the initial stages of development. Later, after the final development of the system it was found that the correlations improved and ranged from 0.76 - 0.81 which were comparable to the tests performed using pubic symphysis. This method was tested by authors on various sample of skeletons including Terry Collection by Murray and Murray in 1991, an archaeological sample from Belleville, Ontario by Saunders et al.,16 in 1992, and sample skeletons from the Grant Collection at the University of Toronto by Bedford et at.,17 in 1993 15-17. Murray and Murray reported that the system developed by Lovejoy et al.,15 in 1985, overestimated age of younger individuals and underestimated age of older individuals in sample of Terry Collection. This was attributed to the differences between age structures of the two samples. Authors concluded that though the method was unreliable to be used as a single ageing technique, it would be useful to estimate age when used in conjunction with other parameters of age estimation.15 Saunders et al.,16 reported that the system was found to be reliable for younger adults, however, it underestimated the age at death for the older adults who were over 45 years. Authors also reported that the intra observer error was as high as 19.3%.16 Bedford et al.,17 in his study reported that the method overestimated age of younger adults and underestimated age for above 60 years old samples. It was concluded that when used systematically along with other parameters of age determination (including pubic symphyseal face), auricular surface can prove to be a valuable tool to estimate age and also help the investigators to conduct the age estimation with increased reliability.17 The features described by Lovejoy et al.,15 appear to develop independently. Hence, for each phase of different features, the age of onset vary, and subsequently the 5-year age categories tend to overlap. In few instances, early-appearing features were still present on the auricular surfaces of older individuals and these features were referred to as “residual.” Considering the occurrence of variations within a single auricular surface indicated that the method described by Lovejoy et al.,15 oversimplifies the changes seen.15 This problem made the application of this method difficult. Buckberry and Chamberlain in 2002 proposed a revised method of estimating age at death using auricular surface of ilium described by Lovejoy et al.15 This revised method recorded age-related stages for different features which were then combined to obtain a composite score to estimate age at death. Buckberry and Chamberlain used features including transverse organization, microporosity, macroporosity, surface texture, and morphological changes in the apex in their study. Based on the degrees of expression of each of the features on the auricular surface, numerical scores were assigned. The use of standardized criteria allowed the examiners to assess the changes objectively. The stages of individual features of auricular surface described by Buckberry and Chamberlain are given in the Tables 2–6.15,18
Score |
Description |
1 |
90% or more of surface is transversely organized. |
2 |
50-89% of surface is transversely organized. |
3 |
25-49% of surface is transversely organized. |
4 |
Transverse organization is present on less than 25% of the surface. |
5 |
No transverse organization is present. |
Table 4 Scoring system for transverse organization by Buckberry and Chamberlain
Score |
Description |
1 |
90% or more of surface is finely granular. |
2 |
50-89% of surface is finely granular; replacement of finely |
3 |
50% or more of surface is coarsely granular, but no dense bone is present. |
4 |
Dense bone is present, but occupies less than 50% of surface; |
5 |
50% or more of surface is occupied by dense bone. |
Table 5 Scoring system for surface texture by Buckberry and Chamberlain
Score |
Description |
1 |
No microporosity is present. |
2 |
Microporosity is present on one demiface only. |
3 |
Microporosity is present on both demifaces. |
Table 6 Scoring system for micro porosity Buckberry and Chamberlain
Reliability tests showed that the revised method had lower observer error and no significant variation was noticed during intra observer error test. It was concluded that this aging technique is valuable, as the auricular surface is resistant to decay when compared to the fragile pubic symphysis. Moraitis et al.,1 tested the revised ageing method using auricular surface developed by Buckberry and Chamberlain in a sample of 120 individuals from Athens Collection. The results concluded that this revised technique was easier to apply when compared to the original method and proved to be a reliable for age estimation on a modern European population. Igarashi and co-workers in 2005 studied 700 modern Japanese skeletal sample and established a new method of age estimation using the morphology of the auricular surface of the ilium. This method was based on the presence or absence of nine and seven features on the auricular surface for a males and females respectively. This method differed from others as observers are not required to examine or measure the degree of development of the feature. After the selection of each parameter estimate, multiple regression analysis with dummy variables were calculated. The authors suggested that this method could give more accurate and reliable age estimates than other especially in the older age ranges.19
Age estimation based on sternal end of fourth rib
McCornick and Stewart in 1988 analysed age changes in the chest plate and suggested that progressive ossification which was distinctive to age and sex, could be seen in the costal cartilages at the sternal end of the rib, both centrichondrally and peristernally. In addition, authors also mentioned other morphological changes noticed in the different parts of the chest plate.20 The sternal end of the rib received the most of the attention by anthropologists to estimate age. Iscan et al.,21 proposed a series of standards which was based on the changes in costochondral junction of the right fourth rib, the walls and surrounding rim, morphology of the pit formation and overall texture and density of the bone. These features were described at each stage as given in Tables 7&8. The metamorphosis of the rib initiates after the completion of the growth at the sternal extremity. The changes include disappearance of the epiphyseal line and pit formation at the billowy surface of the sternal end. The degenerative progression was categorized in to nine phases graded from zero to eight. At phase 0 the smooth and immature rib has flat end with rounded edges which is bordered by epiphyseal line. This surface undergo series of changes like deepening of the pit at the costochondral junction, development of sharp, irregular margins, increase in porosity of bone throughout the life span to reach phase 8. Figure 3 shows the different phases of sternal end of the fourth rib proposed by Iscan et al.22 Authors tested the precision of the method with minimal inter observer error. Though the morphological changes occurring in the rib segment had significant statistical relationships with the age-at-death, factors like sex differences, systemic condition like endocrine disorders, chronic lung disease, intercostal variations, diet and physical activity could influence the aging pattern of the ribs. Authors also pointed that positive identification of the fourth rib, sex differences and intercostal variations were the most vital factors to be considered while using this technique of age estimation.21,22
Score |
Description |
1 |
No macroporosity is present. |
2 |
Macroporosity is present on one demiface only. |
3 |
Macroporosity is present on both demifaces. |
Table 7 Scoring system for macro porosity Buckberry and Chamberlain
Score |
Description |
1 |
Apex is sharp and distinct; auricular surface may be slightly raised |
2 |
Some lipping is present at apex, but shape of articular margin is |
3 |
Irregularity occurs in contours of articular surface; shape of apex |
Table 8 Scoring system for apical changes Buckberry and Chamberlain

Figure 3 Different phases of sternal end of the fourth rib proposed by Iscan et al.21
Martrille et al.,23 in 2007 tested the applicability of four age estimation methods which included pubic symphysis method proposed by Suchey-Brooks, Lovejoy’s auricular surface method, sternal end of fourth rib method put forth by Iscan et al.,22 and lastly age estimation based on the mono-radicular teeth proposed by Lamendin. The study highlighted that there was a tendency of overestimation by all the age estimation methods employed in younger individuals and underestimation in the older age group of individuals. The authors suggested that it is unlikely to estimate age accurately by a single skeletal age marker. Hence, combination of as many as skeletal and dental age indicators should be used.23 Comparing to pubic symphysis method of age estimation Fulginiti et al.,24 also found that the ribs showed lesser variation at any given age. Lower inter observer error was observed by the authors and age estimation based on ribs was considered to more accurate.24 Hartnett also demonstrated a higher correlation (0.66-0.72) between the ribs and the chronological age compared to pubic symphyses.13
Age estimation using cranial sutures
Cranial suture closure pattern has been studied as age marker by many investigators for nearly a century. Unfortunately, its reliability is still debated as it does not give a precise age. This is because of high intra sutural variability seen along its length. It is rare to see completely fused cranial sutures in the second decade of life or patently unfused sutures in the elderly. None the less when combined with other methods of age estimation it could be an important age indicator as the fibrous joints between the skull bones fuse progressively with increasing age. Based on the degree of closure of both the endocranial and ectocranial sutures various scoring systems ranging from completely open to complete obliterated have been developed. It was believed by Todd and Lyon that endocranial closure could be more reliable due to the phenomenon of “lapsed union” i.e. a suture may show union on the endocranial surface with failure of closure at the external surface.25 Meindl and Lovejoy mentioned that the critical forensic questions involve the degree and nature of synostosis occurring in the later years and not the regularity of closure at the early adult years. Meindl and Lovejoy in 1985 limited their study to ectocranial sutures for the reason being that the ectocranial activity is more closely related to extreme age for which new forensic standards are most needed. This method is preferred to use due to its practical approach. They examined 10 specific sites which they divided into the vault system and the lateral-anterior system. Among the two systems, the lateral-anterior proved to be the better predictor of age. Composite scores were obtained from the sum of site scores from both the systems and correlated to stages with age range and mean age.26
The sites which were studied by Meindl and Lovejoy are
The sites are shown in the Figure 4. The vault system consisted of the first seven sites and the last three, combined with pterion and mid coronal were referred to as the lateral-anterior system. Each site was scored as given in the Tables 9&10.26 Meindl and Lovejoy concluded that the relation between degree of cranial suture closure and age is general. Key and co-workers in 1994, studied three different methods of age estimation using cranial suture closure in 183 individuals from Spitalfields, London. In their study they compared techniques proposed by Acsadi and Nemeskeri, Meindl and Lovejoy and Perizonius. Thirty six ectocranial and 18 endocranial sites of the cranial sutures were examined. Scores for cranial suture closure was allocated as given in the.26–29 Though age estimation from endocranial suture closure is applicable between wide age limits only, Acsadi and Nemeskeri maintained that endocranial suture closure is an important age marker, especially when used with other age indicators. Meindl and Lovejoy categorised the ectocranial sutures into two sets namely,
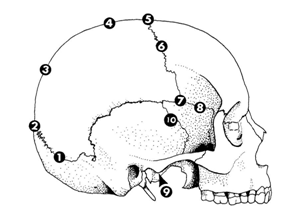
Figure 4 Ten sutures sites studied by Meindl et al.26
Score |
Description |
0 |
Open; there is no evidence of any ectocranial closure at the site. |
1 |
Minimal closure; some closure has occurred. This score is |
2 |
Significant closure; there is a marked degree of closure but |
3 |
Complete obliteration; the site is completely fused. |
Table 9 Scoring of cranial sutures closure (by Meindl and Lovejoy)
Score |
Description |
0 |
Suture with no trace of closure |
1 |
Incipient closure |
2 |
Closure in progress |
3 |
Advanced closure |
4 |
Completely fused suture |
Table 10 Martin’s system of scoring of cranial suture
Authors found that for both lateral anterior and the vault systems, Meindl and Lovejoy’s sample mean ages were significantly lower than the Spitalfields’ sample mean ages (p< 0.01). This indicated delayed cranial suture closure in the Spitalfields sample than Meindl and Lovejoy's sample. This was attributed to the inter-population difference between the Meindl and Lovejoy sample and the Spitalfields crania.26,28 Perizonius defined two separate systems
The Y system was based on the endocranial suture sites, whereas the O system was based on both ectocranial and endocranial suture sites. The results were obtained from the composite suture closure scores.27,29 Results concluded that the Acsadi and Nemeskeri technique, which was based on endocranial sutures sites, could be used to distinguish between young and middle aged individuals. However, this technique did not give any information for individuals above 50 years of age in the Spitalfields sample. The O system of Perizonius method demonstrated no relationship with age in older individuals and Y system showed accuracy of only 47.2 per cent. Meindl and Lovejoy and Perizonius techniques which were based on ectocranial sutures, were found to be significantly influenced by sexual dimorphism.26–29 In 1998, Nawrocki studied 100 crania from the Terry Collection with age ranging between 21 to 85 years. The author scored 27 cranial landmarks which included 16 ectocranial, 7 endocranial, and 4 palatal sutures. These 100 crania were sub grouped into 4 having 25 samples in each. Sutures on the vault were scored following Meindl and Lovejoy i.e. 1 cm segments along the cranial sutures were scored on a four-point scale. To improve the correlation, group-specific equations were developed.26,30
Nawrocki’s results suggested that the equations tested performed better among males and on average it tend to underestimate the age with a moderate correlation between the summed cranial suture closure score and the age.30 Zambrano in 2005 tested Nawrocki’s equations among 388 samples from three different universities namely the University of Indianapolis, the University of Tennessee at Knoxville, and the University of New Mexico at Albuquerque. The author summarized that correlations between cranial suture closure and age were comparatively lower in the modern sample than the Terry sample. It was also concluded that correlations were seen higher in the vault system for females whereas in case of males, correlations were higher in the lateral-anterior system. Zambrano’s study reaffirmed the correlation of cranial suture closure with age and reported that the pattern and/or rate of cranial suture closure was influenced by sex.31
Apart from the above mentioned traditionally used four skeletal traits for evaluation in adult skeletal aging, there are other variables cited in the literature which forensic anthropologists used. These variables could be used in conjunction with the above mentioned conventional methods.
Medial clavicle epiphyseal fusion
The medial clavicle epiphysis can be potentially used an age marker in aging young adults as it is last to fuse. The beginning of fusion of medial clavicle is seen with the onset of puberty and is not completed until the late 20s. Different scoring systems were proposed by various investigators. Langley-Shirley and Jantz in 2010 compared the ferred was to as “no union” which explained that no remnant of the flake was fused to the shaft. The second phase was depicted as “beginning union”. During this phase the epiphyseal flake sho5 phase system to 3 phase system.32 McKern and Stewart proposed 5 phase system, where phase 1 reuld have commenced the fusion to the medial articular surface, however not more than 50% of the surface should be covered by the flake. The third phase is the “active union” phase where, the epiphyseal flake covers 50% or more of the surface and fusion occurs actively. The authors considered active union at the epiphysis when the flake appeared clearly as a separate entity and some space occurred between the bone surface and edges of the flake. The fourth phase is the “recent union” phase. During this phase the flake completely fuses to the shaft. However, fusion scar and ⁄ or small bony nodules could be seen as the trace of fusion event on the outer rim of the medial surface. Finally the fifth phase was referred to as “complete union”. This score was given when the trace of the fusion event was completely absent and the articular surface was quiescent.33 In the three-phase system phase 2 to phase 4 of McKern and Stewart system i.e. beginning, active, and recent union were clubbed into one phase named as “fusing”. Therefore, the three-phase system was outlined as “unfused” (phase 1), “fusing” (phase 2) and finally “fused” (phase 3). Langley-Shirley and Jantz compared these two-phase systems in clavicles from 1289 individuals. The observer error tests suggested three phase scoring system to be least subjective and it also retained the accuracy levels. The authors concluded that while estimating age for modern individuals the results underscored the importance for usage of modern standards.32,33
Acetabulum surface
Surviving the post depositional environments, the durable os caxae is one of the valuable tool for age estimation. Rissech et al.,34 proposed a method to determine age of adult males based on traits of acetabulum and considered to be an effective age marker.34 Rouge Maillart et al.,35 modified the method of age determination based on the acetabulum and the auricular surface of coxal bone.35 The authors identified four age related traits including acetabular fossa, acetabular rim, lunate surface and the apical activity and combined it with Buckberry and Chamberlain’s technique.35,36 They combined the scores obtained by the two methods and obtained a composite score. The results suggested an average correlation between score and actual age and confirmed the reliability of the method. Calce and Roger tested this method among 100 adult males. The applicability of this technique resulted in an inaccuracy of 8 years. It was observed that the inaccuracy was least for above 40 years of age.37 Calce suggested that though the method produced encouraging results, whenever possible it should be combined with multiple age indicators for better accuracy of age estimation.38
Age determination using proximal femur
The architecture of proximal femur allows it to deal with various stresses it is subjected to. These stresses can originate internally and/or externally of the hip joint. Based on the intensity of these stresses, resorption of trabeculae of the femur occurs in an ordered pattern. Walker and Lovejoy in 1985 compared age estimation methods based on the morphological changes in proximal femur, clavicle, proximal humerus and calcaneus. They proposed an eight-staged method for the morphological changes occurring proximal femur on aging. The authors demonstrated that the secondary trabeculae were gradually lost which was followed by the loss of primary trabecular lines seen within the proximal shaft, femoral head and neck. The authors stated that highly variable pattern of bone loss was observed during the third and fourth decades of life.39 The Figure 3 shows the age-related changes occurring in the femur.
Osteoarthritis
Arthritic change in the skeletal parts is another probable age indicator. These changes are specific to the individual. Stewart in 1958 introduced a five staged system which described the vertebral osteoarthritic patterns in aging adults.40 This classification was tested by Snodgrass in 2004 and he reported that there was a significant correlation between the patterns of arthritic changes and the chronological age of the individual. However, the author warned regarding higher degree of variations seen in the osteophytic activity. Hence, it was suggested that osteoarthritic could be used in general for identifying the upper and lower boundaries of age.41 Factors such as sex, lifestyle, health conditions and culture influence the rate of degeneration and render it inconsistent.
Age estimation using histomorphometry
Histomorphologic studies including analyses of osteons involved in bone remodelling, Haversian canals count and cortical thickness are also used to estimate age. Apart from being invasive, the microscopic methods are laborious, time consuming, require special equipment and specialized training. These methods should be employed with caution as high level of inter observer inconsistency is often noticed specially while conducting osteon counts.3
Factors affecting accuracy of skeletal age assessment
The aging process is underlined by various internal and external factors such as gender, ancestry, chronic illness, nutritional conditions, life-style and genetic makeup of the individual.42,43 These factors are can become potential source of bias in age estimation. Further, both within and between individuals and populations great variations are seen which can influence the biologic ageing and subsequently affect the results of the age estimation. The major problem faced is the human senescence. This is determined by the characterized metabolic disorders in individuals. The continuous interactions between gene-culture-environment contributes to the individual’s senescence.44 It is often shown that the age estimation methods used, prove to be inaccurate and less reliable when applied to the population other than the results obtained from the original study sample population. Hence, it is recommended to use population specific data for more reliable age estimation.14
Asymmetry
Biological asymmetry can occur due to the variation witnessed in the development and degeneration process across the skeletal elements.43 Expression of age characteristics is influenced by duration of maturation period and the environmental factors.45,46 Hence, a prolonged maturation period and a spectrum of highly variable environmental factors, provide greater probability for biologic asymmetry, resulting in inaccuracy of aging of skeletal remains.
Selection of appropriate age estimation method
One of the major factors for the appropriate choice of age estimation for adults is the skeletal part available for the analysis. The quality of these skeletal remains also determines the method. Hence, the choice of skeletal age estimation method would differ between a well preserved complete body (skeletonized or decomposed) and fragmented body parts.44 Baccino and co-workers highlighted two step procedure when complete body is available. In the two step procedure, methods employed are complementary and not combined. The authors reported that based on the hypothesis that a single method would not be relevant to the entire life span, combining the methods leads to a fundamental error as these methods are derived from different reference samples. One of the limitations to use two step procedure includes the lack of one or two of the age indicators.44 Baccino and co-workers compared seven methods of age estimation from mature skeletal remains in a sample of 19 French autopsy individual of known age at death. One of the objectives this study was to determine the relative value of using a multifactor method to single method. The authors strongly suggested that “comprehensive approaches to age estimation that consider multiple age indicators are superior to isolated methods”.47 Hence, they supported the conclusion derived by Saunders et al.,16 that the most appropriate approach for age estimation is the one which considers all available age indicators.16
Statistical analysis
The selection of appropriate statistical analysis for skeletal age estimation in adults is often debated. This is primarily because of two issues, firstly, difference between the statistical approaches used in different skeletal age estimation methods, and secondly, the difference between the data available from the original reference sample and the applicability of this reference standards to an individual not belonging to the reference group.48 Therefore, in order to reduce bias related to statistical methodology, investigators are challenged to develop a standardized statistical analysis in performing age estimation.44
Among the various age indicators cited in the literature, the hip joint which renders two independent sites i.e. the auricular surface and the pubic symphysis for age estimation in adults, is considered to be most accurate age marker. It should be kept in mind that the bias involved in the process of age estimation using any technique is inconsistent throughout the lifespan of the individual, characteristically increasing beyond fifth decade of life. In case of advanced age and especially when the skeleton is fragmented, histological analysis of the bone can be suggested as a viable alternate method of age estimation. Estimating age in adults remains a challenging task to the forensic anthropologists because of the complexity and individual variations seen in the aging process and the gamut of environmental factors influencing the same. As repositories of skeletons with known age, sex and ancestry are becoming increasingly rare, and because the existing collections subsume individuals from historical periods, an alternate reference data for modern populations must be developed. Further, with the advancements in the forensic anthropology field and the need to continuously improvise and validate methods, the conventional approach for age estimation in adults may be challenged. Advancements like radiographs, computed tomography and magnetic resonance imaging are already being utilized as complementary source of data.49 The researchers are now confronted with development of new population specific standards using the recent advancements for age estimation.
None.
None.

©2017 Priya. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.