eISSN: 2575-906X


Review Article Volume 3 Issue 6
1Department of Environmental Health Sciences, University of Ibadan, Nigeria
2Department of Community Health and Primary Health Care, University of Lagos, Nigeria
3Department of Physiology, Ladoke Akintola University, Nigeria
Correspondence: Sokan Adeaga Adewale Allen, Department of Environmental Health Sciences, Faculty of Public Health, University of Ibadan, Ibadan, Nigeria, Tel +234-7089-261568
Received: September 09, 2019 | Published: November 21, 2019
Citation: Allen SAA, Ree AG, Ayodeji SAM, et al. Secondary inorganic aerosols: impacts on the global climate system and human health. Biodiversity Int J. 2019;3(6):249-259. DOI: 10.15406/bij.2019.03.00152
Secondary Inorganic Aerosols (SIA) is of core relevance in climate, public health and ecosystem. Aerosols can be categorized as primary, when it is directly released from sources, and termed secondary, when subsequently formed in the atmosphere from chemical processes involving a set of precursor gases. SIA usually consist of a mixture of components: Sulfates, nitrates, ammonium, black carbon, sea salt, mineral dust etc. They affect the climate explicitly by scattering and absorbing of solar radiation and implicitly by modifying the clouds properties. The principal parameters that governed the environmental and health effects of aerosols particles are their size, concentration, chemical composition, and structure. These parameters, however, are temporally and spatially highly variable. Multicity studies had shown that associations between SIA and morbidity and mortality differ across locations, with this discrepancy attributed in part to diversity in size effect and chemical composition. The review concludes that SIA due to their size and chemical composition has a great impact on the climate system and human health. Also, the short lifetime of SIA (days/weeks) implies that their effects are more regional and less persistent into the future than those of the long lived greenhouse gases (GHG). In devising policies to curb health effects, simultaneously, climate impacts should be considered, and vice versa. Thus it is becoming more and more lucid that regional air quality and global climate issues are intricately linked to one another.
Keywords: secondary inorganic aerosols, atmospheric chemistry, climate system, health effects, greenhouse gases, air quality
The particulate matter (PM) can be defined as a multi-component system that consists of solid or liquid particles which are suspended in the atmosphere and release from anthropogenic and natural sources. These particles plays a vital role in the Earth’s radiation budget,1 dimming of atmospheric visibility,2 participate in acid deposition,3 pose huge threats to cultural heritage4 and can be linked with numerous pulmonary ailments, cardiovascular problems and decline in life-expectancy.5 PM may be classified as into primary and secondary. Primary PM are those that emitted explicitly from origins while secondary PM are created in the atmosphere by chemical reactions involving a group of precursor gases. This latter fraction is, mainly produced by series of physical processes and chemical reactions involving sulfur dioxide (SO2), nitrogen oxides (NOx), ammonia (NH3) and several number of volatile organic compounds (VOCs), which may combine with hydroxyl radical (OH), ozone (O3) and other reactive molecules creating both secondary organic aerosol (SOA) and secondary inorganic aerosol (SIA) respectively.6
The core secondary inorganic aerosols (SIA) components in PM include Ammonium (NH4+ ), nitrate (NO3− ) and Sulfate (SO42- ) occurring as ammonium nitrate (NH4NO3) and ammonium sulfate ((NH4)2SO4) respectively, which are formed, by the neutralization of nitric acid (HNO3) and sulfuric acid (H2SO4) with ammonia7 as depicted by the below equations:
NH3 + HNO3→NH4NO3 -----------------------------(eq 1)
NH3 + H2SO4→(NH4)2SO4 -------------------------- (eq 2)
The neutralization of nitric acid is generally being prevail over by the sulfuric acid neutralization,8 but secondary nitrates and/or sulfates production is strongly dependent on numerous chemical and micro-climatic conditions, viz air temperature and humidity, concentrations of atmospheric oxidants, gaseous precursors levels, and the characteristics of pre-existing aerosols.9,10 In Europe, a huge proportion of the mass of fine particulate matter (with aerodynamic diameter less than 2.5μm, PM2.5), ranging from 11% to 35% and from 1% to 24 %, are contributed by the non-marine nitrate and sulfate respectively,11 and these particulates plays a vital function in the acidity of aerosol and its detrimental impacts on materials, ecosystems and man health. Sea salts mainly compose of Na+ and Cl−, affect PM2.5 levels and acidity greatly in coastal areas. In Europe even when the coarse mode (particles with aerodynamic diameter greater than 2.5μm) constitute about 95% of the total mass of marine aerosols,8 sea salt in PM2.5 ranges from less than 1% (in remote continental areas) to 11% (in Atlantic zones).11
The impact of SIA on climate and human health is largely governed by size and composition, and both factors differ greatly from one particle to another. For climatic effects, the quantity of particles (of intermediate size) is far more crucial than the mass. This is negates their impacts on ecology which are primarily determined by the mass concentration. They arise from both natural and man-made sources. In term of health implication, SIA is a complicated mixture; thus the toxicity of each chemical component varies in places and time. Evidences have abounded on the relationship between aerosols and adverse health effects through acute term studies, such as time series studies and case crossover studies.12,13 These studies rely on daily variations of the concentrations of PM components. Thus, it is still onerous to established the fact whether certain PM is of huge public health interest over the others.
Nevertheless, subtleties persist in numerous aspects, with diversification for optimal policy portfolio. Several of these involve the SIA property, whose impact in human health and more especially in climate change is attributed with the greatest ambiguity. The objective of this review is to enunciate the nature, formation and atmospheric chemistry of secondary inorganic aerosols (SIA) and their impacts. It also provides an in-depth exposition of secondary inorganic aerosols in the context of the climatic change and health impacts, and how it is envisaged to modify to change in the future; with techniques that can be employed to control aerosols pollution.
Overview of aerosols
Origin
An aerosol can be described as a disperse system with air as the carrier gas and having a solid or liquid disperse phase or a mixture of both. In Atmospheric Science, it is general to use the term “aerosol” just for the solid or liquid particles without paying attention to the carrier gas. Solid or liquid particles suspended in the atmosphere are called aerosols. They can either be released explicitly into the atmosphere, in such case referred to as primary aerosol e.g. black carbon, dust and sea salt), or they can be created by condensation in the atmosphere, thus termed secondary inorganic aerosol e.g. ammonium, nitrates and sulfates.
Size
Virtually, all aerosol characteristics and their impacts are dependent on size. The size of the aerosol governs their health effects, their impact on clouds, their interaction with radiation, and the rate at which they settle to the ground (and thus atmospheric lifetime). Their size varies from a few nanometers (1 nm = one millionth of a millimeter) to tens of micrometers (1μm = one thousandth of a millimeter). Aerosols are usually classified based on their equivalent spherical diameter, despite not all particles are spherical. The diameters of aerosol particles (AP) range from 1 nm to several hundred µm. Thus, it is very crucial to classified atmospheric aerosol particles in different modes as shown in Figure 1 according to their diameters: Nucleation mode (10-3µm – 10-2µm); Aitken mode (10-2µm - 0.1µm); Accumulation mode (0.1 µm - 1µm); and Coarse mode (particles > 1 µm). Aerosol particles greater than 10µm are commonly termed as giant aerosol particles, while all particles less than 1 µm are often referred to as fine particles.
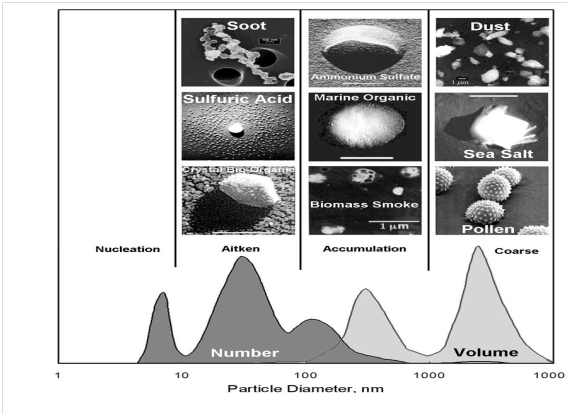
Figure 1 Typical shape of the aerosol number and volume size distribution, showing the various size classes (‘modes’) as well as example pictures of some archetypical aerosol particles. 1 nanometer (nm) = 0.001 micrometer; upper right value should read 10000 nm = 10 micrometer.
Number and mass
The number of tropospheric aerosols particles concentration varies from several tens or hundreds per cubic centimeter of air in distant regions, to greater than hundred thousand or a million per cubic centimeter in polluted surroundings or after a strong nucleation event. The size distribution is often used to describe the amount or volume concentration as a function of particle size. The smaller particles are generally described in term of the number of particles whereas the larger ones are described by volume (and thus mass). The environment reflects the sources, sinks, and transformations of the particles; and it is a main determinant of the shape and size distribution of the aerosols.
Formation
Several vapour molecules coalesce together to create a new particle. This process is often referred to as nucleation. Coagulation is another process by which aerosol particles collide together. This process is particularly efficient, after a nucleation occurrence, during which hundreds of thousands of new particles can be formed. Only a minute fraction of these small particles grow to attain large size capable of influencing the climate. Nucleation result in the formation of a larger proportion of small particles (with diameters less than 0.1 micrometer) to be formed in the atmosphere. Suitable variables such as high relative humidity; atmospheric mixing processes; low temperature; high solar radiation intensity; presence of ions; a strong source of condensable vapor; and low pre- existing aerosol concentration are necessary for nucleation to occur.14
Transformation
Particles undergo several transformation processes in the atmosphere viz: coagulation, oxidation, condensation, evaporation, and polymerization because they cannot maintain their original configuration for a very long duration. A typical example is carbon or soot particle which undergoes oxidation at the edges, when more water retaining species condense on its surface, thus it formed a liquid mixed particle, with traces of black carbon, inorganic compounds and organic species. Aerosol particles dependent on size only persist in the atmosphere for several days to weeks before selecting to the floor. Particles with longest atmospheric lifetime are those of intermediate size (of a few hundred nanometers, aptly named the accumulation mode).
Formation mechanisms and constituents of secondary particles
Secondary particles are not emitted explicitly but are created in the atmosphere by series of chemical reactions involving inorganic or organic gas-phase components. Nucleation is a process by which secondary particles are created, this involved low volatile molecules condensing to form solid or liquid matter. There are two unique kinds of process namely heterogeneous and homogenous nucleation. Majority of secondary particles are formed by ‘heterogeneous’ nucleation in which newly created substances grow in size by condensing onto existing particles. While in homogenous nucleation, newly-created molecules of low vapour pressure and lack of substantial pre-existing particles (which is suitable for heterogeneous nucleation), will congregate to form wholly new substances.15
The mechanism of production of secondary inorganic aerosol (SIA) is comparatively well lucid; despite some mechanistic details still remain vague.15 SIA consist mainly of ammonium nitrate (NH4NO3) and ammonium sulfate ((NH4)2SO4), with some sodium nitrate (NaNO3). These compounds arise from the transformation of precursor nitrogen oxides (NOX) and sulfur oxides (SOX) in the atmosphere to nitric and sulfuric acids, which are then neutralized by atmospheric ammonium (NH4+). Ammonia is the precursor to atmospheric ammonium. Combustion process gives rise to both SOX and NOX. The principal source of NH3 emissions is from agriculture, viz urea and uric acid decomposition in husbandry waste.15
The formation rate of secondary particles occurs very tardily; the overall oxidation rates of NO2 and SO2 are approximately to 1% per hour and 5% per hour respectively. The tardiness of these reactions, couple with the fact that the resulting particles are minute and therefore have a comparatively long atmospheric lifetime. This infers that secondary particles can travel several kilometres downwind from the source of their precursors. Emission sources of NOX typically contribute to secondary nitrate observed tens to hundreds of kilometres downwind, while SO2 sources typically contribute to secondary sulfate noticed hundreds to thousands of kilometres downwind. As a result, there is a significant even distribution of secondary PM on a regional scale, with lesser discrepancies between rural and urban settings than for primary particles.16 Several researches have indicated that secondary particles can add immensely to the concentrations of PM, particularly at background locations such as discussed in the extensive review by the United States Environmental Protection Agency (USEPA) Figure 2.17
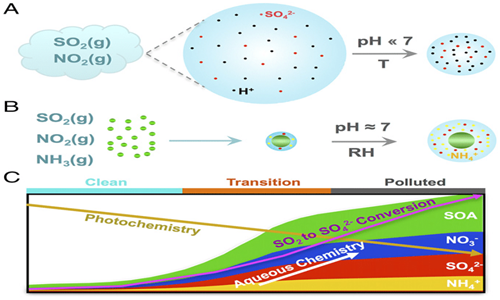
Figure 2 Formation mechanism and constituents of secondary particles.18
Impacts of atmospheric aerosols
Since the inception of urbanization sprawl and industrialization, atmospheric particles are among one of the major culprits affecting urban air pollution. Environmental pollution has become a serious threat with continuous increase in global economic growth. Atmospheric particles is a complex interwoven system of organic and inorganic substances, which exist as solids and liquids particles in the atmosphere, and they extended over a distant range from a few nm in diameter to greater than or equal to 100μm. They also differ in chemical composition, shape, and optical properties.19 It is well established that the chemistry, physics, and optical properties of secondary organic and inorganic aerosols are closely associated to homogenous and heterogeneous-phase chemical reactions, which consequently led to a myriad of environmental degradations, including ozone layer depletion, air quality deterioration, smoke-fog related accidents and acid rain formation. Aerosols particles are intuitive atmospheric trace substances that can be detected through sensual perception; they are affect visual field and are inimical to the respiratory systems of human and animals.20–24
Atmospheric aerosol pollution can pose a huge hazard and there have been periodic records of vital accidents arising from aerosol pollution.23,25 Aerosols emitted from metropolitan air pollution usually consist of several complex organic chemicals viz polycyclic aromatic hydrocarbon (PAHs), agricultural chemicals, volatile organic compounds (VOCs) and fumigants.26–29 The physiological impacts of these chemicals on human health are of great significant. Therefore, are interconnected to human existence. Another important type of aerosol impacting on the environment is dust. Dust particles can be travelled from their source of emission to long range distance and impacts the environment and well-beings of population residing in other places.30,31 It widely acknowledges that dust can influence photochemical oxidation and ocean environment and neutralize acid rain.32
Overview and dynamics of climate change
Weather is defined as the reflection of the atmospheric status which includes its precipitation, wind, humidity, temperature etc. over hours to weeks. It is influenced by the cryosphere, hydrosphere and terrestrial activities, couple with the atmosphere to constitute what is termed the ‘climate system’.33–35 Climate, in its widest definition, is the statistical overview of the status of the atmospheric system. Climate change can be view as a variation in the statistical characteristics of the climate system that persist for several decades or more, usually minimal of 30 years. These statistical properties include variability, extremes and averages. Climate change may arise from natural occurrences, such as internal variability in the climate system, volcanoes or variation in the Sun’s radiation or arising from man-made influences viz land use changes and pollution of the atmosphere.36
Within the past one decade or thereabout, there seems to be a wider acceptance that climate change poses one of the most challenging threats to contemporary life. By and large, this can be attributed to publications of overwhelming scientific evidences by several international agencies and organizations such as the declaration by Intergovernmental Panel on Climate Change (IPCC) in early 2007 that climate change is anthropogenic (i.e. result of greenhouse gas emission arising from human activities) and others related-organizations viz The Royal Society,37 NRC38 and American Meteorological Society.39 The continuous rising in greenhouse gases (GHG) emission and its consequential effect of global warming has reached an alarming level. The height of this fear was reached in the 19th century when the global mean temperature attained 0.6oC. Consequently, a further increase in temperature of only 1.4oC is deemed permissible. This is based on the anticipation that the mean long term rise in temperature which must not be surpass, except if detrimental effects are envisage, could proceed or (maintain) at the rate of 0.2oC per decade.40–42
Natural processes such as ocean current shifts, variation in solar output and others phenomenon influence the Earth’s climate. Nevertheless, they are not suffice, to offer succinct explanation to the rise in temperature we observed in last half-century43 as indicated in Figure 3. Most of the warming of the past half century has been attributed to anthropogenic greenhouse gases emissions as depicted by Figure 4.44 Several man-made activities are responsible for the release of greenhouse gases into the atmosphere; the two principal sources are: land use change and combustion of fossil fuels which contribute over 95% of global CO2 emissions. Others sources include livestock rearing, industrial activities, crops fertilization and landfill gases.45
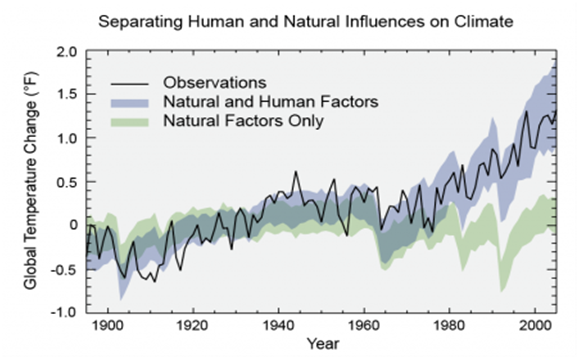
Figure 3 Models that account only for the effects of natural processes are not suffice to explain the warming observed over the past century. Models that also account for the anthropogenic greenhouse gases emissions are able to explain this warming.43
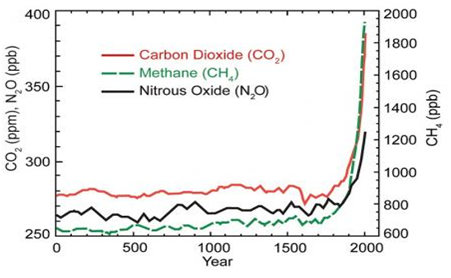
Figure 4 This graph shows the increase in greenhouse gas (GHG) concentrations in the atmosphere over the last 2,000 years. Increases in concentrations of these gases since 1750 are due to human activities in the industrial era. Concentration units are parts per million (ppm) or parts per billion (ppb), indicating the number of molecules of the greenhouse gas per million or billion molecules of air.44
The magnitude of this change will depend on the quantity and frequency of mitigation of greenhouse gas emissions. During the 21st century, global warming is forecasted to persist and climate changes are likely to aggravate. Scientists have used climate models to project various aspects of future climate, including ocean acidity, ocean level, rainfall, temperature, snow and ice. Depending on future greenhouse gases emissions and the dimension of climate responds, average global temperatures are projected to rise globally by 0.5°F to 8.6°F by 2100, with a probably increase of at least 2.0°F for all scenarios besides the one representing the most aggressive reduction in emissions of greenhouse gases.46
Climate effects of secondary inorganic aerosols
Climate is influence by factors often referred to as ‘radiative forcing’. This denotes a change in the radiation budget of the earth, which consequently leads to either cooling or warming. Aerosol particles can impact climate in numerous ways, as schematically represented in Figure 5.47
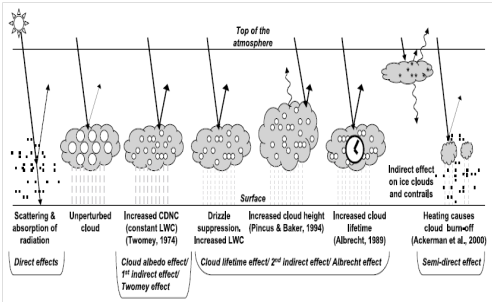
Figure 5 Several ways in which aerosol particles affect climate. The small black dots represent aerosol particles; the larger open circles represent cloud droplets, and the asterisks stand for ice crystals. Perturbed clouds have more (and thus smaller) droplets because of the availability of more aerosol particles on which to condense. CDNC = cloud droplet number concentration; LWC = liquid water content.47
Direct effects
The climatic effects of aerosol particles themselves are often refer to as direct effects.48 Aerosol particles are capable of scattering and absorbing (in the case of black carbon) solar radiation. Since a substantial percentage of the scattered radiation will be reflected back into space, this results in atmospheric cooling. The most effective of the PM at backscattering of solar radiation are the intermediate sized particles (from 0.1 to 2 micrometer diameter). A key parameter governing the strength or intensity of the explicit effect is the number of these intermediate sized particles. Black carbon absorbs solar radiation, which results in atmospheric warming. If cloud droplets are being incorporated with black carbon rich particles, the lens effect of the water aggrandizes the absorption, and may result into evaporation of the cloud droplet attributed to the local heating. This is phenomenon is often termed the semi-direct effect. Additional warming occurs when black carbons are deposited on ice and snow leading to a decline in the surface albedo. Predominantly, aerosol compounds causes cooling except for black carbon, since they do not absorb solar radiation. To a lesser extent mineral dust can absorb solar radiation.
Indirect effects (cloud formation)
The role of aerosols in cloud formation is termed their indirect effects on climate.49 Aerosol particles function as cloud condensation nuclei (CCN) around which cloud droplets can form. The effectiveness of the CCN is directly proportional to size of the aerosol particle. These are illustrated in Figure 6 by the reflective nature of the stratus clouds; and Figure 7 that show the process involved in the formation of the CCN. Under most conditions obtainable in Western Europe, particles must have a diameter greater than around 0.1 micrometer in order to participate in the formation of cloud. The more the availability of the aerosol particles (CCN), the greater the cloud droplets formed. The average size of the cloud droplets will be smaller, since the available water has to be distributed among all the droplets. Nevertheless, the cumulative total surface area of all the droplets is larger, and the resulting cloud is more reflective (meaning, it has a higher albedo; this is the first indirect effect, or cloud albedo effect). Such a ‘perturbed’ cloud, will not rain out quickly as it consist of more but smaller droplets. Consequently, the cloud stays longer (this is the second indirect effect, or cloud lifetime effect). CNN activity is greatly affected by particle size rather than by chemical composition.50 The main parameter governing the strength of the indirect effect is the number concentration of particles greater than ~0.1 micrometer (as long as they are not water repellant/hydrophobic).

Figure 6 The indirect effect is depicted by aerosol emissions from shipping. The elevated aerosol concentration in the wake of a ship is responsible for more reflective (i.e. whiter) nature of the stratus clouds.52
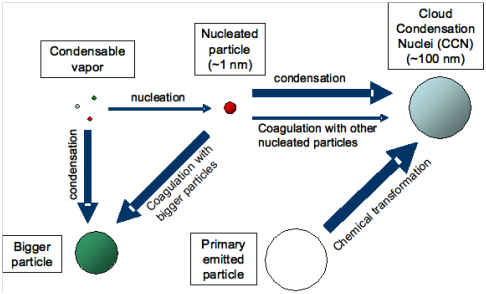
Figure 7 Several factors govern the extent to which nucleation contributes to the number of cloud condensation nuclei (CCN).14
Ice crystals can be form on a small subset of particles, hence behaving as ice nuclei (IN). The ice crystal formation usually occurs via freezing of a liquid droplet, a process called ice nucleation. A typical good ice nucleus is mineral dust. There is also evidence that black carbon can take part in ice nucleation, though the evidence is yet inconclusive.51 If this assertion is true, then the increased burden of black carbon as a result of man-made activities could have led to a rise in the ice crystals number, which is effective at instigating rainfall. This would lead to a decreased cloud lifetime and cloud cover, resulting in climate warming. Nevertheless, this is still a speculative hypothesis and has not been either proven or measured.
The precise impact of having increased number of CCN in the atmosphere is dependent on numerous factors. When viewed from above, clouds reflect sunlight and emit infrared radiation to space, and so exert a cooling effect. Assessing from below, clouds absorb and re-emit infrared radiation back to the surface (similarly to greenhouse gases), and so exert a warming impact. The global net effect is a cooling, but depending on conditions, this can differ in place and time. High clouds generally cause warming of surface, although low clouds generally cool. This is attributed to the fact that high cirrus clouds usually being optically thin; reflect only a minute fraction of the incoming solar radiation. Moreover, they re-emit radiation of low energy level due to their low temperature, so less energy is release to space and more retains in the atmosphere. During the night, clouds cause warming, due to absence of solar radiation to reflect; and absorption and re-emission of infrared radiation from the surface. Cooling generally dominates during the day but this usually depends on altitude.
Uncertainty
The resultant effects of aerosols on climate are worth noting, nevertheless also greatly vague. There are numerous reasons why the uncertainty in aerosol climate effects is so enormous vis-a-vis to that of long-lived greenhouse gases:
The principal greenhouse gases lifetime (excluding tropospheric ozone) is measured in years (e.g. methane) or even centuries (e.g. carbon dioxide), while aerosol particles have a short lifespan of about several days to maximum of a few weeks in the atmosphere, before they precipitate to the ground.
Consequently, the aerosol concentration differs remarkably in location and time, and estimates of (variation in) its global concentration field is very imprecise.
The aerosols in the atmosphere are a conglomerate of particles that differ greatly in chemical composition and size. Particle size and composition are key factors that govern their effects on climate (as well as the health).
Nucleation processes of aerosols formation in the atmosphere are poorly elucidated.
Aerosols impact on clouds is vague, due to the fact that cloud dynamics and microphysics have numerous dependencies and are abstruse.
Although our understanding of the role of aerosols in climate system is rising at a very minimal rate, nevertheless this will continue to take precedence over the ambiguity of expected climate changes into the near future (few decades). Futuristically (multiples decades to centuries), expected climate change is more likely to be governed by greenhouse gases rather than by aerosols, since the plateau on the former is deemed higher than on the latter.
Health impacts of secondary inorganic aerosols
The major secondary inorganic particles that are formed from gaseous primary pollutants are sulfate and nitrate. Due to their relative abundance, minimal hazard and high solubility in human body, these secondary inorganic particles have been assumed to be less inimical compared to the primary combustion-derived particles.53
Epidemiological studies
In 2009, the EPA integrated science assessment noted that secondary sulfate had strong correlation with both pulmonary and cardiovascular health effects in acute epidemiological studies. At that instance, several studies began to focus on the cardiovascular effects of PM constituents and sources rather than on pulmonary effects. Since the integrated science assessment, there had been copious epidemiological evidences on the short-term effects of sulfate on both cardiovascular54 and respiratory55–57 hospital admissions; two studies have connected cardiovascular mortality to sulfate.54,58 New evidence has shown the relationship between daily increases in ambient sulfate and physiological changes linked to cardiovascular diseases, such as endothelial dysfunction and ventricular arrhythmias.59,60
It worthwhile noting that sulfate just like black carbon, is linked with a number of other constituents from fossil fuels combustion, such as organic compounds and transition metals. In several aspects, nitrates and sulfates are closely tight to ammonium and hydrogen. It is pertinent to considered sulfate as an indicator of detrimental constituents from coal and oil combustion. On the contrary, the situation may be more complicated: it has been reported that sulfate also enhance iron solubility,61 which may expedite the particles harmfulness. The follow-up study of the Harvard Six Cities Study reported that there was no significant rise in the magnitude of the impacts of PM2.5 mass over time, in spite of the relatively higher drop in sulfate concentrations, vis-a-vis PM2.5 mass during the study period.62 This surmise that sulfate contribute significantly to the ambient PM toxicity. Howbeit, it is still uncertain whether elimination of SO2 (a precursor for sulfate) from the emissions of coal and oil combustion would result in a significant mitigation in the health effects linked with these sources. Also, it is believed that if the formation of new particles of ammonium sulfate or sulfuric acid occurs in the atmosphere, it may serve as a condensation sink for primary and secondary organic components.
Nitrate is another indicator of emissions from combustion processes, including traffic exhausts which are rich in oxides of nitrogen. In a mortality study, conducted in Seoul, Republic of Korea, there was substantial evidence linking nitrate and more strongly for ammonium to cardiovascular, but not pulmonary diseases.58 On the contrary, two studies on hospital admissions found evidence associating nitrate to pulmonary disease but not cardiovascular.55,56 It is important to note in these studies, that cardiovascular admissions were not linked to sulfate, corroborating the fact that present evidence for sulfate is still weak. Only one recent study has explored relationship between chronic exposure to nitrate and health. In a study conducted in California,40 both sulfate and nitrate were associated with cardiopulmonary mortality. In multi-pollutant models, organic carbon and sulfate also showed strong significant relationship. Also in the United States, multiple analyses of prospective cohort studies have linked mortality with long-term exposure to sulfate.63
Clinical and toxicological studies
Since the review by Schlesinger et al.,53 no new relevant information or evidence has emerge which concluded that secondary inorganic aerosols have minimal biological potency in well functional humans or animals or in animal models with limited compromise at environmental exposure levels. Reiss et al.,64 reported that toxicological studies provides weak or no support for a causal relationship between particulate sulfate compounds and a risk to health at ambient concentrations. In same light, there is limited toxicological evidence to support a causal association between particulate nitrate compounds and excess health risks. Nevertheless, it cannot be precluded that the cations associated with nitrates and sulfates (such as transition metals, acidity marked by hydrogen cations), nor absorbed components (such as organic particles) may be the underlying cause of the strong associations between sulfate and health effects, because ammonium bisulfate or ammonium sulfate can be regarded as a relative low toxic material, in relation with polycyclic aromatic hydrocarbons or transition metals. Hitherto there is no publication in toxicological studies that investigated the role of nitrates (or sulfates) in the complex mixture of PM; currently, the fact still remains that bioavailability of other components, such as metals are greatly influenced by these secondary inorganic components.
Expected future trends
Sulfate has gain wider attention than nitrate as a dominant aerosol component, though awareness of its relevance is gradually rising. When there is excess ammonia and sulfate aerosol is fully neutralized, then ammonium nitrate aerosol will be forms. The quantity of nitrate aerosol (and its climate impact) is therefore dependent on both NOx emissions and ammonia. Due to the high emissions of ammonia, Netherlands has a higher amount of nitrate aerosol than anywhere else in the world. Consequence to the implicit dependence on sulfate neutralization, a decline in sulfate aerosol may result to a rise in nitrate aerosol (if ammonia is the limiting factor for ammonium nitrate formation and not NOx), which could partially balance the climate effects.
The burden of aerosol relies to a large extent on the gaseous precursors emissions, such as ammonia (NH3), volatile organic compounds (VOC’s), sulfur dioxide (SO2), and nitrogen oxides (NOx). Developed nations have mostly achieved emission reductions of these pollutants, although it is also expected that the third world countries, primarily for public health justifications would follow suit. In nearby decades, it is forecasted that SO2 emissions will continue to rise in India and China, but decline in Europe and North America. Also, it is predicted that emissions in other part of the globe will not change significantly. Similar pattern of predicted change is valid for NOx.65 Most VOC’s are released in the biosphere, and its future progression is vague. Some researchers have hypothesized that temperatures rise will result to greater biogenic emissions of e.g. terpenes, which would contribute immensely to the formation of secondary organic aerosol (SOA).66 Also it is envisage that direct aerosol emissions, such as those arising from automobiles exhaustion are to be further curbed. Two conditions are momentous for predicting future climate impact from anthropogenic emissions. Firstly, is the short chemical life span of most climatically active aerosols vis-a-vis CO2, and secondly, the possibility of decreasing emissions through mitigation options. In view of these conditions, it is probable that the relative contributions of CO2 to climate change in the future (timescale of decades to centuries), will be greatly aggrandizing.65
Techniques and devices for controlling aerosols
There are numerous methods of controlling aerosols viz: Adsorption, Absorption, Reduction System and Condensation System.
Adsorption: Adsorption involves the interaction between gaseous particles and the surface of a solid adsorbent. In this phenomenon molecules from a gas or liquid become attached extrinsically to a surface. This form of interaction is usually weak and reversible. The most commonly used industrial adsorbents are silica gel, activated carbon, and alumina, due to their enormous surface areas per unit weight.
Absorption: Aerosols that are soluble in aqueous liquids can be removed by absorption. This is one of the key mechanisms employed in the removal of acid gas compound (e.g. sulphur dioxide, hydrogen chloride, and hydrogen fluoride) and water soluble organic compounds. All absorption processes operate optimally at low temperature of gas and liquid. Gas and vapour phase contaminants are the most soluble under cold conditions.
Reduction systems: The destruction of oxides of Nitrogen (NOx) compounds released by combustion processes is primarily carry out by reduction systems. These systems can be sub-divided into two namely: selective non-catalytic reduction systems (SNCR) and selective catalytic reduction system (SCR). In both types of systems, a chemically reduced form of nitrogen is injected into a gas stream to react with oxidized nitrogen compounds, namely NO and NO2. The reaction between the reduced and oxidized form of nitrogen result in molecular N2 the major constituent of clean air. SNCR and SCR are believed to be effective for NO and NO2 removal.
Condensation system: Condensation systems are exclusively employed in industrial process effluent gas steams for the recovery of organic compounds present at moderate to high concentrations. The most common type of condensers are those using cooling water in direct contact or indirect contact vessels. Refrigerators and cryogenic systems are used primarily in the high efficiency recovery of high value contaminants. Condensation systems reduced the contaminant concentration in the gas stream to a concentration equivalent to the vapour pressure of the compound at the operating temperature of the condenser.
Control devices for particulate pollution include:
Electrostatic precipitator: An electrostatic precipitator (ESP) is a particle control device that employs electrical force to eject the particle out of the flowing gas stream and onto collector plates. The ESP impacts electrical charges on the particle, causing them to be attracted to oppositely charge metal plates situated in the precipitator. The particles are removed from the plates by “rapping” and collected in a hopper located below the unit. The removal efficiencies for ESP are highly variable; nevertheless it has a removal efficiency rate of 99% for very small particles alone.
Fabric filter: Fabric filter, or baghouses, is used for removing dust from a gas stream, by passing the stream through a porous fabric. The fabric filter is efficient at removing fine particles with greater than 99% efficiency in most applications. Nevertheless, one of the demerits with fabric filter is that high-temperature gases often have to be cooled before contacting the filter medium.
Venturi scrubber: Venturi scrubbers employ liquid stream to remove solid particles. In this device, gas laden with particulate matters passes through a short tube with flared ends and a constricted middle. This constriction causes the gas stream to accelerate when the pressure is increased. A water spray is directed into the gas stream either prior to or at the constriction in the tube. The discrepancy in velocity and pressure arising from the constriction causes the particle and water to mix and combine. Venturi scrubbers are efficient in removing minute particles, with removal efficiencies up to 99%. However, a major disadvantage with the use of this device is the production of wastewater.
Cyclones: Cyclones operate by collecting relatively large size particulate matter from a gaseous stream through the use of centrifugal forces. Dust laden gas is made to rotate in a decreasing diameter pathway forcing solids to the outer edge of the gas stream for deposition into the bottom of the cyclone.
Secondary Inorganic Aerosol (SIA) due to their size and chemical composition has a great impact on the climate system, human health and ecosystem. Multicity studies (both clinical and epidemiological studies) had shown that associations between secondary inorganic aerosols (SIA) and morbidity and mortality differ across locations, with this discrepancy attributed in part to variation in chemical composition and size effect.
Climate change arises from numerous factors, which may be man-made and natural. The global warming that has impacted the earth for over the past ~150 years is as a consequence of interaction of many factors, the most crucial one being anthropogenic greenhouse gases, which cause the earth to trap more infrared radiation than usual. Nevertheless some of this warming is masked by aerosol cooling. Aerosol particles impact the climate explicitly by the absorption and scattering of solar radiation and implicitly by modification of clouds properties. Aerosols are short-lived which implies that their impacts are more localize and does not persist into the future vis-à-vis the long lived greenhouse gases. Thus, it is envisaged that there would be reduction in the global aerosol concentration but at different pace in differ regions. The outcome of such global aerosol concentration reduction would be a more intense warming, as the hideous greenhouse warming trend will be more exposed. Conclusively, this review opined that in devising policies to attenuate the health hazards posed by aerosols, it becomes imperative to take climate impacts into consideration, and vice versa. Hence, it becomes lucid that regional air quality and global climate issues are intricately interwoven.
The authors appreciate the efforts of all those who had contributed immensely to the field of environmental health studies.
The authors declare there are no conflicts of interest.

©2019 Allen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.