eISSN: 2575-906X


Research Article Volume 2 Issue 5
1Facultad de Ciencias Agrarias, Politécnico Colombiano Jaime Isaza Cadavid, Colombia
2Facultad de Ciencias Agrarias, Universidad Nacional de Colombia, Colombia
Correspondence: Sandra B Muriel, Facultad de Ciencias Agrarias, Politécnico Colombiano Jaime Isaza Cadavid, Carrera 48 #7-151, Medellín, Colombia
Received: July 31, 2018 | Published: September 11, 2018
Citation: Osorio VA, Muriel SB, Torres JMC. Morphoagronomic characterization of Tamarindus indica L. in orchards of tropical dry forest from Antioquia (Colombia). Biodiversity Int J. 2018;2(5):396-403. DOI: 10.15406/bij.2018.02.00092
Tamarind (Tamarindus indica L.) is a plant genetic resource, which is important for the tropical dry forest economy of Antioquia (Colombia); despite being an introduced species, the tamarind has adapted to the region, becoming part of its cultural identity. Nevertheless, the lack of scientific studies of tamarind has shown this crop to be an underutilized species. The local community, in its traditional knowledge and experience, recognizes two types of tamarind: the acid one, and the sweet one. A phenotypic diversity of 50 trees with fruit of an acid flavor and 48 trees with a sweet flavor were evaluated, based on 26 morph-agronomic descriptors. The Gower distance, and the UPGMA method were used to determine the diversity and make the dendrogram. In order to compare both phenotypes, the Bayes methodology was used, and the highest posterior density intervals of 95% of probability were obtained. Results show that the acid phenotype has trees with pyramidal crowns, that has a rough cortex and the sweet phenotype cortex is brittle; that the fruit of sweet phenotype are smaller with lighter pulp and seeds than the acid phenotype; also, the acid phenotype shows more weighty seeds than sweet phenotype. Although no differences in the content of sugar in the pulp (Brix degrees), it was found the acid phenotype is 1.8 times more acid than the sweet phenotype. Tamarind trees from the region have a morphoagronomic diversity of 32%, which is important to collect germplasm of this promissory and multipurpose species and to identify the best individuals for propagation.
Keywords: morphological diversity, sweet tamarind, acid tamarind, Tamarindus indica, promissory specie
Tamarind (Tamarindus indica L.) is an evergreen tree, it belongs to the Caesalpinioideae subfamily (Fam: Fabaceae). It is thought that the origin of this species is tropical Africa and it has become naturalized in many areas around the world.1 It is a plant genetic resource that has a great potential, introduced to America in the 18th century, likely by the Spanish and Portuguese in the early years of African slaves’ trade.2,3 According with IUCN, Tamarindus indica has been evaluated as a least concern (LC).1 The tamarind is a multipurpose tropical tree, being the mostly used fruit around the globe because it is used in sodas, sweets, meat condiments, liquors and in the elaboration of syrups. Despite the high and diverse use value by rural communities for a long time,4 only recently this species has aroused interest among the scientific community due to its medicinal application as a potential source of natural antioxidant5,6 and other functional properties.7,8 For Colombia, tamarind is an introduced species; however it is well adapted to the tropical dry forest of Antioquia and The Caribbean region, because of its rusticity and adaptation to long periods. The production of Antioquia is centered in Santa Fe de Antioquia and Sopetrán; these municipalities are part of a touristic rout of the region where tamarind is offered as juice, sweets or fresh fruit to the tourists; It is also considered as an ornamental and shadow tree. The tamarind crop is essential to the local community, nevertheless its management is carried out with a little technical knowledge and a limited technological application.9‒12 The tamarind trees of the region are long-lived and with low yields; in addition, the participation of Antioquia in the national production has decreased to less than half from 68.8% in 2009 to 23.2% in 2014. This has a relation with a change of the use of the soil and, in general, to the local lack of interest of the administration in the conservation of the traditional fruit of the region. The Local population has traditionally classified tamarind according to its qualitative characteristics, differentiating two types: the “Acid Tamarind” and the “Sweet Tamarind”; this differentiation is made by their pulp, flavor and other morphological characteristics such as fruit shell color (epicarp), affirming that the sweet tamarinds are darker colored.
There are no reports of the morphological variation of tamarind in Colombia; however, three tamarind varieties are reported in India, identified by the fruit size, the number of seeds and the pod color. The first one is known as East Indian, and it produces big pods with six to twelve seeds; the second one is identified as West Indian, and it has short pods with four seeds, approximately.3 Two tamarind types are reported in Mexico: a big pod type and a small pod type, they are differentiated from each other by the number of seeds per fruit and the size of the pod. The small pod type has from one to five seeds and it is from three to eight cm long; the big pod type has from one to ten seeds and it is from 3 to 15cm. long. Markets prefer the big pod tamarind type because it is commercialized as fresh fruit, whereas the small pod type is better in the elaboration of sweets and sodas. There is a type of tamarind in Thailand known as “Makham waan” and another one established in the south of Florida (USA) called Manila Sweet.3,13 In Venezuela, farmers differentiate two tamarind types: “Rabo é mono” and “Pipe é mono”. A study13 about the floral morphology of both phenotypes found that Rabo é mono has larger floral branches with less flowers, with very elongated atrophied ligule shaped petals (2.0mm) and ovaries with a surface of greater pubescence and larger trichomes; whereas the petals of the Pipe é mono’ type are triangular and 1.0mm long.14 The bunches of the Rabo é mono type are distributed along and across the crown, whereas the bunches of the Pipe é mono ones are located in the center of the crown.14 According to the authors, these morphological characteristics allow easy phenotype recognition in the field.14 In order to morphologically differentiate the tamarinds properly, Fandohan et al.,15 suggest that locally perceived quantitative and qualitative descriptors should be combined, such as the pulp color and the flavor. Recently, a study determined a genetic diversity of 32 tamarinds plus trees using inter-simple sequence repeat (ISSR) markers, finding a genetic diversity enough for a clonal propagation program, and a habitation program.16
The aim of this research was to know the existing phenotypic diversity of T. indica in traditional orchards of tropical dry forest in Antioquia, and establish whether there are differences between the acid and the sweet phenotypes, through morpho-agronomic characterization.17‒19 The most characteristics that define a phenotype correspond to a morphological description of the plant and its architecture,18 but other interesting morphological characters are included, which are important from an agronomic, genetic improvement and marketing point of view.
The evaluated tamarind trees were located in farms in the municipalities of Sopetrán and Santa Fe de Antioquia (Table 1), which are in the Tropical Dry Forest life zone of Antioquia with an average temperature of 24ºC.20 The Specific descriptors for T. indica were elaborated using documented Descriptors for Tropical Fruits21 as a guide. Furthermore, interviews to farmers and harvesters of the region, and field and laboratory observations were carried out; the descriptors guide was also adjusted and validated to discard descriptors with non-existing or extreme variation in the same individual. All trees were distributed between 619 to 740m of altitude (Table 1).
No. |
Municipality/Village |
Farm |
Geographic coordinates |
Altitude (m.a.s.l) |
Number of trees |
1 |
Sopetrán/La Miranda |
El Ensueño |
6º30’ 44.5” N 75º45’ 13.2” W |
675 |
9 |
2 |
Sopetrán/El Llano |
La Ceiba |
6º29’ 39.9” N 75º45’ 41.5” W |
660 |
22 |
3 |
Sopetrán/El Llano |
Los Comuneros |
6º29’ 45.3” N 75º45’ 55.0” W |
664 |
11 |
4 |
Sopetrán/Guaimaral |
Casa Roja |
6º26’ 41.5” N 75º46’ 59.8” W |
593 |
7 |
5 |
Sopetrán/El Rodeo |
El Abrujo |
6º30’ 39.8” N 75º47’ 44.9” W |
490 |
4 |
6 |
Santa Fe/El Espinal |
Cotové-UNAL |
6º32’ 06.1” N 75º49’ 49.6” W |
553 |
16 |
7 |
Santa Fe/Zona urbana |
Zona urbana |
6º33’ 36.0” N 75º49’ 26.4” W |
622 |
12 |
8 |
Santa Fe/El Tunal |
El Común |
6º37’ 13.0” N 75º49’ 23.5” W |
567 |
15 |
9 |
Santa Fe/San Nicolás |
Urb. Palmar del Cauca |
6º28’ 57.1” N 75º49’ 10.3” W |
505 |
2 |
|
|
|
Total sampled trees |
|
98 |
Table 1 Characteristics of sampled sites for morph agronomic characterization of Tamarindus indica L
Field sampling
98 trees distributed in nine sites of commercial plantations, and dispersed trees in urban and rural zones were sampled; 50 phenotype acid trees, and 48 phenotype sweet trees. The characterization process began in the field where in situ measures of eight descriptors were made: the crown shape (CS), the stem branching height (BH), the diameter at breast height (DBH), the cortex texture (CT), the number of leaflets pairs (NLP), the petiole length (PL), the spine length (SpL) and the phenotype (FNT) according to the criteria of the formers and harvesters. In all the cases, the pulp was consumed to confirm the sweet or acid flavor; however, the final decision came from the local people. Sampling was carried out in a tamarind harvesting period, from December 2014 to February 2016. At least 30 mature and sane fruits per tree were evaluated; then they were packed in plastic bags, labeled with origin, tree number and phenotype data, and consequently taken to the laboratory.
Laboratory evaluation
Seeds and fruits characterization were carried out in the Botany and Physiology Laboratory of the Politecnico Colombiano Jaime Isaza Cadavid in Medellin, Antioquia. The seeds and fruit descriptors were evaluated: the weight of 30 fruits (WF), the weight of the epicarp of 15 fruits (WFE), the weight of pulp plus seeds of 15 fruits (WPS), the weight of the seeds of 15 fruits (WFS), the weight of the pulp of 15 fruits (WFP), the length of the pod (PL), the width of the pod (PW), the number of seeds per fruit (SF), the total soluble solids (TSS), the length of the seed (SL), the width of the seed (SW),the shape of the seed (SS), the red component of the color of the fruit (RPu), the green component of the color of the fruit (GPu), the blue component of the color of the fruit (BPu), the red component of the color of the epicarp (REp), the green component of the color of the epicarp (GEp) and the blue component of the color of the epicarp (BEp). In order to determine the weight of the fruit, the weight of 30 fruits (WF) descriptor was used per tree and, from this, the rest of measures were taken.
To measure the sugar content of the tamarind pulp, the total soluble solid (TSS) descriptor was used, it corresponds to the saccharose concentration at a 20ºC temperature and it is expressed in Brix degrees (ºBrix). Because of this, a portable analogue refractometer was used and five measures per tree were taken. To contrast the TSS results. The acidity percentage of the acid phenotype, and the sweet phenotype were compared using a titration with NaOH (0,05M) solution, and phenolphthalein as indicator. Samples of 50.0g of tamarind pulp were taken, with eight repetitions per each phenotype. The color was measured with a LT Lutron colorimeter, RGB-1002 model, using a red, a green and a blue (RGB) color scale. The Color values are expressed in a three-digit combination with a minimum and a maximum values of 0 and 255, respectively. The combination of the digits determines a specific color. The easy RGB® (2014) online color calculator was used to observe the given RGB color.
Phenotypic diversity analysis
The Gower22 distance was used in order to analyze both quantitative and qualitative variables. The distances were used to generate the dendrogram based on the pairs grouping with the unweighted arithmetic mean (UPGMA) method. To determine the dendrogram adjustment, the cophenetic correlation coefficient (CCC) proposed by Sokal & Rohl23 was calculated. Subsequently, the grouping distance was determined visually, and the groups were established; all of this was made in R (R Core Team 2016) statistical environment.
Comparison between two phenotypes
The Bayesian methodology was used with the implementation of the Markov chains in Monte Carlo simulation (MCMC) generating 1x106 chains and taking one of every ten samples for their parameters estimation. The burn-in period was 5000 samples, and thus the effective length of the chain was 1x105. This was calculated using the MCMCglmm24 package in the R environment. The mean of the a posteriori distribution of the parameter was used as a Bayes estimative. Furthermore, the highest posterior density intervals (HPD) of 95% of probability was obtained using the CODA25 package from the R environment. In order to know if there was any difference between phenotypes measures, the difference between each chain sample was calculated. The respective mean and HPD of 95% probability were obtained. For the qualitative variables, the proportion of individuals of each category in each phenotype was determined and such proportions were compared using the Bayesian methodology described for quantitative variables. Binom and BayesianFirstAid26 packages in the R environment were used.
26 descriptors were established, 22 quantitative and four qualitative for T. indica. The descriptors showed the discrimination among the trees, and from samples taken inside each tree. The flower descriptors were discarded since they were observed in an earlier evaluation that most flower characteristics varied a lot in the same individual or did not varied at all among the trees. Furthermore, the flowering time was not homogeneous even for trees in the same farm.
In general, the weight of 30 fruits (WF) showed the highest standard deviation (DE=80.2), indicating that it is the most variable descriptor. In addition, the pulp and the seeds weight (WFP and WFS, respectively) presented the highest CV (47.1% and 48.5%, respectively), suggesting that they are very variable descriptors. Branching height (BH) is another highly distributed characteristic (21–233cm) with a variation in height (CV=40.0%). Moreover, the number of leaflets pairs (NLP) and the total of soluble solids (TSS) descriptors varied a little and had the lowest CV (12.8% and 13.3%, respectively). Fruit of 86.8mm long (PL) were found, which corresponds to similar sized pods described in literature27 about fruit of 82.0mm long with six seeds per fruit. In the south of The US, fruit from 80 to 150mm long were found,9 which leads the fruit of this study to the inferior limit of this range. The fruits presented four seeds per fruit (SF), which coincides with the East Indian variety that has small pods and four seeds per fruit.3 Nearby the western of Antioquia fruits can be described with small pods as they are in the inferior limit of reported sizes range, and coincides with the number of seeds of the East Indian variety. The pod length and seed number are two characteristics important for predicting yield and for evaluation and selection of elite trees.28
The qualitative crown shape (CS) descriptor was a low representative of this analysis because it is homogenously distributed among the individuals. The 60% of the trees showed brittle cortex texture (CT), this could be because the most of the evaluated trees were old; more than 30 years old, and their cortex were not as rough as they were brittle. Although the tamarind seeds presented various shapes, oval shaped seeds predominated (60%) (Figure 1).
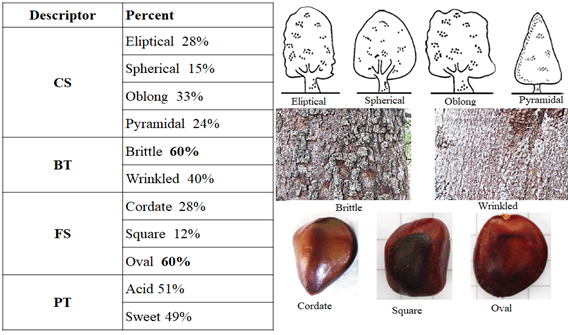
Figure 1 General tamarind (Tamarindus indica) frequencies of qualitative descriptors in tropical dry forest of Antioquia (Colombia).
The found cofenetic correlation of the value of the coefficient (CCC) was 0.605, indicating the obtained dendrogram by the UPGMA method represents 60% the information of the matrix of the Gower distances. This is an adequate value to know the relation between the studied variables23. Five groups were obtained from grouping analysis, three well defined and two atypical groups. The dendrogram cutting height was established at 0.327, which means that evaluated individuals differ in 32%, approximately. This also corresponds to an important diversity value that stimulates the search of productive interesting materials (Figure 2) (Table 2). Groups were characterized as follows (Table 3).
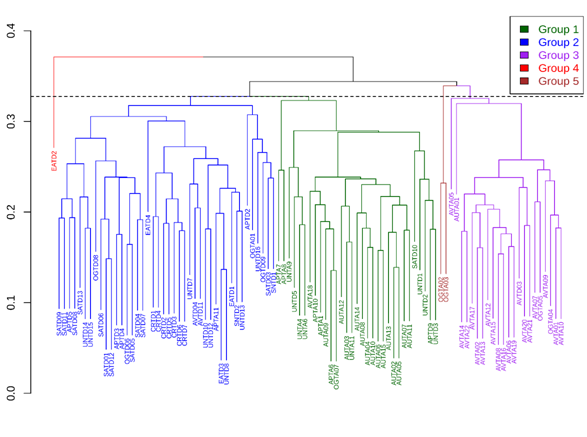
Figure 2 Dendogram of Tamarindus indica L population of the tropical dry forest of Antioquia (Colombia), based on morpho-agronomic data, generated by UPGMA grouping method and Gower distance.
No. |
Quantitative descriptor |
Symbol |
Unit (Si) |
Min. |
Max. |
Media |
Standard Deviation |
Coefficient of variation CV (%) |
1 |
Branching height |
BH |
cm |
21 |
233 |
118 |
47.3 |
40 |
2 |
Diameter at breast height |
DBH |
cm |
10 |
64 |
32 |
11.5 |
36 |
3 |
Weight of 30 fruits |
WF |
g |
59 |
458 |
262.5 |
80.2 |
30.6 |
4 |
Weight of 15 epicarps of the fruits. |
WFE |
g |
19.5 |
76 |
40.1 |
11.3 |
28.2 |
5 |
Weight of pulp plus seeds |
WPS |
g |
28 |
176 |
91 |
30.3 |
33.3 |
6 |
Weight of 15 seeds of the fruits. |
WFS |
g |
7 |
99 |
45 |
21.8 |
48.4 |
7 |
Weight of 15 pulps of the fruits |
WFP |
g |
13.5 |
125 |
46 |
21.7 |
47.1 |
8 |
Number of paired leaflets. |
NLF |
Unity |
9 |
18 |
13.6 |
1.7 |
12.8 |
9 |
Petiole length |
PL |
mm |
3 |
10 |
6.8 |
1.5 |
22.7 |
10 |
Spine length |
SpL |
mm |
4 |
10.3 |
7.1 |
1.1 |
15.9 |
11 |
Pod length |
PL |
mm |
42.4 |
148.8 |
86.8 |
16.8 |
19 |
12 |
Pod width |
PW |
mm |
8 |
33.7 |
22.4 |
3.2 |
14.2 |
13 |
Number of seeds per fruit |
SF |
Unity |
1 |
8 |
4.0 |
1.3 |
36.6 |
14 |
Total soluble solids |
TSS |
ºBrix |
15 |
55 |
44.9 |
6 |
13.3 |
15 |
Seed length |
SL |
mm |
7.4 |
23.5 |
14.4 |
2 |
14 |
16 |
Seed width |
SW |
mm |
6.8 |
19.3 |
12.3 |
1.8 |
14.5 |
17 |
R-Pulp parameter |
RPu |
R |
16 |
123 |
40,9 |
13.5 |
33 |
18 |
G-Pulp parameter |
GPu |
G |
15 |
78 |
26.3 |
7.4 |
27.9 |
19 |
B-Pulp parameter |
BPu |
B |
12 |
55 |
21.1 |
5.8 |
27.3 |
20 |
R-Epicarp parameter |
REp |
R |
29 |
185 |
80.9 |
26.1 |
32.3 |
21 |
G-Epicarp parameter |
GEp |
G |
20 |
148 |
60.8 |
20.9 |
34.4 |
22 |
B-Epicarp parameter |
BEp |
B |
16 |
126 |
48.7 |
18.7 |
38.4 |
Table 2 List of descriptors and general statistics of evaluated quantitative variables for Tamarindus indica L
Group |
1 |
2 |
3 |
4 |
5 |
||||||||||
Number of individuals |
31 |
42 |
22 |
1 |
2 |
||||||||||
Gower distance Average |
0.26 |
0.29 |
0.25 |
0 |
0.23 |
||||||||||
Descriptor |
Media |
DE |
CV |
Media |
DE |
CV |
Media |
DE |
CV |
Media |
DE |
CV |
Media |
DE |
CV |
BH |
125.2 |
55.1 |
44.0 |
123.9 |
48.5 |
39.1 |
92.6 |
30.1 |
32.5 |
165.0 |
- |
- |
87.5 |
38.9 |
44.5 |
DBH |
30.8 |
8.2 |
26.5 |
36.1 |
12.3 |
34.0 |
26.4 |
11.5 |
43.6 |
46.0 |
- |
- |
20.0 |
1.4 |
7.1 |
WF |
281.2 |
53.9 |
19.2 |
228.2 |
80.7 |
35.3 |
314.3 |
57.9 |
18.4 |
360.0 |
- |
- |
74.5 |
21.9 |
29.4 |
WEF |
43.6 |
10.3 |
23.7 |
34.6 |
10.2 |
29.5 |
47.1 |
8.8 |
18.8 |
38.2 |
- |
- |
23.5 |
3.5 |
15.0 |
WPS |
96.2 |
19.8 |
20.6 |
78.8 |
32.2 |
40.9 |
110.3 |
21.4 |
19.4 |
141.8 |
- |
- |
30.3 |
3.2 |
10.5 |
WSF |
53.0 |
18.3 |
34.6 |
31.3 |
14.0 |
44.7 |
63.0 |
19.9 |
31.6 |
47.7 |
- |
- |
9.0 |
2.8 |
31.4 |
WPF |
47.5 |
18.8 |
43.4 |
43.2 |
25.4 |
53.6 |
47.5 |
14.8 |
31.2 |
94.1 |
- |
- |
21.3 |
0.4 |
1.7 |
NLF |
13.1 |
1.4 |
10.9 |
13.8 |
2.1 |
14.9 |
13.4 |
1.8 |
13.5 |
15.0 |
2.6 |
17.0 |
13.4 |
2.2 |
16.6 |
PL |
7.6 |
1.2 |
16.3 |
6.5 |
1.6 |
24.1 |
6.0 |
1.3 |
22.0 |
5.5 |
0.9 |
15.4 |
7.4 |
1.1 |
14.5 |
SL |
7.3 |
0.9 |
12.3 |
7.2 |
1.2 |
16.1 |
6.8 |
1.3 |
19.6 |
7.6 |
0.2 |
2.4 |
7.0 |
1.0 |
14.8 |
PL |
86.5 |
16 |
18.5 |
73.3 |
19.3 |
26.4 |
92.8 |
21.4 |
23.1 |
86.1 |
16 |
18.6 |
75.9 |
17.1 |
22.5 |
WL |
22.1 |
3.3 |
15.1 |
22.1 |
3.0 |
13.5 |
23.6 |
3.1 |
13.2 |
20.1 |
3.4 |
16.7 |
22.9 |
3.0 |
13.2 |
SF |
3.9 |
1.2 |
29.9 |
3.1 |
1.2 |
39.5 |
4.0 |
1.4 |
33.5 |
5.4 |
1.4 |
31.2 |
2.9 |
1.2 |
39.8 |
TSS |
42.2 |
6.1 |
14.4 |
45.7 |
6.2 |
13.4 |
47.2 |
3.9 |
8.3 |
48.4 |
2.0 |
4.0 |
42.9 |
3.5 |
8.1 |
SL |
14.1 |
1.9 |
13.4 |
14.1 |
1.6 |
11.6 |
15.8 |
2.2 |
14.1 |
11.0 |
1.1 |
10.3 |
13.7 |
2.2 |
16.0 |
SW |
12.3 |
1.7 |
13.5 |
12.2 |
1.7 |
13.8 |
12.8 |
1.9 |
15.1 |
8.7 |
0.4 |
4.7 |
11.8 |
2.2 |
19.0 |
RPu |
45.3 |
11.5 |
25.4 |
36.7 |
11.7 |
31.8 |
41.3 |
12.6 |
30.5 |
20.3 |
4.0 |
19.9 |
68.3 |
28.7 |
42.0 |
GPu |
27.6 |
5.4 |
19.5 |
25.2 |
6.3 |
25.2 |
25.7 |
8.4 |
32.5 |
18.7 |
3.5 |
18.8 |
41.0 |
19.1 |
46.5 |
BPu |
22 |
5.3 |
24.3 |
20.6 |
4.7 |
22.9 |
20.4 |
6.6 |
32.6 |
17.0 |
3.0 |
17.7 |
29.5 |
13.4 |
45.3 |
REp |
87.2 |
21.5 |
24.7 |
70.9 |
23.2 |
32.8 |
93.0 |
29.6 |
31.9 |
38.7 |
1.2 |
3.0 |
82.7 |
18.7 |
22.6 |
GEp |
64.2 |
17.1 |
26.6 |
53.7 |
19.7 |
36.7 |
70.7 |
23.3 |
32.9 |
31.7 |
1.5 |
4.8 |
67.0 |
13.3 |
19.9 |
BEp |
50.3 |
16.4 |
32.6 |
43.5 |
17.9 |
41.2 |
56.7 |
20.3 |
35.7 |
26.3 |
1.5 |
5.8 |
57.8 |
12.4 |
21.4 |
Table 3 Quantitative variables description according to formed groups using UPGMA, evaluated in 100 Tamarindus indica L. individuals.
Group 1
Formed by 31 individuals, 81% of them were of acid phenotype, so the phenotype is the most discriminant variable of the group, it was not of the fruit origin. This group showed individuals with a brittle cortex texture (B), an oblong shaped crown trees (Obl), cordate shaped seeds (66%), leaves with larger petioles (PL=7.6cm), an homogenous weight of fruits (WF of 50% of the individuals was between 249.0 and 310.0g), a low percentage of pulp (31.0%), four seeds per fruit and light brown colored epicarps (Rep=84.5, GEp=61.0 and BEp=47.0). The mean DBH was 31.0cm, the mean length and the width of the fruit was of 86.5mm and 22.1mm, respectively. The Branching height (BH) was the most variable descriptor in this group (CV=44% and SD=55.0cm). This Group had a similar individual weight of fruits, and phenotype.
Group 2
Formed by 42 individuals, with a 95% of the sweet phenotype. The Individuals came from eight different sampling sites, meaning that their origin was not a determinant factor in grouping. The trees presented brittle cortex and diverse crown shapes, being oblong the most popular (38%), and the shaped the most seeds had were oval (75%). This group presented trees with DBH of 36.0 cm, half of the individuals with the thickest stems (DBH>33.0cm), which can mean that this group was formed by the oldest trees, possibly very appreciated by the farmer(s). The trees with the smallest fruit (PL=78.0mm and PW=22.0mm), and with a variable low weight (SD=80.65 and WF=228.2 g). This can be related to the lack of management that is given to the sweet phenotype by farmers, transformers and marketers do not prefer it because in the production of sweets more pulp is required than when they use acid tamarind. The Fruits with three seeds per fruit and dark brown colored epicarps (REp=60.4; GEp=52.7 and BEp=42.0) which agreed with the local people who affirms that the recognition of the sweet tamarind fruits was by its dark color.
Group 3
Formed by 22 individuals where 21 (95.5%) were of the acid phenotype, with a new phenotype as the predominant grouping variable. However, its origin was a discriminant factor in this group because 86% of the trees are located in the farm “La Ceiba” in the municipality of Sopetran. The trees in this group presented a pyramidal (73%) crown shape (CS), oval shaped seeds (91%) and rough cortexes (95%). This group has the youngest trees (15 years old), which agreed with the perception of the former that rough and brittle cortexes leads to the youngest and the oldest trees, respectively. Furthermore, this group presented very thin stems (DBH=24.6cm). The individuals are characterized by yielding the biggest (PL=100.0mm and PW=24.0mm), and the heaviest (WF=314.0g) fruits, with four seeds per fruit, with a light brown-greyish colored epicarp (Rep=90.0, GEp=68.1 and BEp=56.3). An inverse relation between the age and weight of the trees was observed, heavier fruits were obtained from younger trees; this is the case on “La Ceiba” farm, where trees grow in a silvopastoral production system with a sprinkling irrigation and two annual fertilizations, it is a unique condition of the sampling sites.
Group 4
Formed just by one sweet phenotype individual. It is an atypical group formed by the individual EATD2 coming from a tree located on the farm “El Embrujo” in Sopetrán. It presented the thickest stem (DBH=46.0cm) and the highest branching height (BH=165.0cm), which could be related to the age of the trees because the farm “El Embrujo” happens to have the oldest trees (40 years old).
This tree had the heaviest fruits (WF=360.0g) with a high pulp percentage (52.0%). The individuals were separated by the weight of the fruit (WF), the size of the seed (PW and PL), DBH, BH and the variety of the color. The EATD2 is an interesting individual due to the promising characteristics of its pulp exploitation because it is an old tree but still produces heavy and high pulp fruit content.
Group 5
It is an atypical group formed by two individuals of acid phenotype, OGTA02 and OGTA03, that have the same origin. They presented the thinnest and most homogeneous stems among the groups (DBH=20cm, CV=7.1%), the lightest fruits (WF=74.5g) with the lightest brown colored pulp (RPu=68.3, GPu=41.0 and BPu=29.5). Both individuals were separated due to their low weight of fruit, their seeds and pulp values, and the lightest color of pulp.
In general, their origin was not a differentiating factor when separating the individuals (except for group 3), which could be related to the environmental conditions and similar management in all sampling sites. Without two atypical 4 and 5 groups, its fruit weight coincides with the pod length and pulp weight. Its bigger pods are heavier and have more pulp. This agrees with Fandohan et al.,15 who concluded that the size of the fruit is a good predictor of the pulp content in T. indica. The sugar content (TSS) in tamarind pulp presented low CVs in all the groups, from 8.1% to 14.4%, indicating that this characteristic was very homogenous and did not contribute in any way to the grouping.
Acid and Sweet phenotype comparison
The acid tamarind individuals had bigger fruits (PL=84.0mm) with heavier pulp plus seeds weight (WPS=99.4g) and heavier and larger seeds (WSF=51.4g and SL=14.6mm) than the sweet tamarind had, (Table 4). In India,29 it was found fruit of 200.4mm long, which evidenced that fruit found were very small (84.0mm) compared to those of the Indian fruit. Moreover, in Mexico there are two tamarind varieties according to the size of their fruits they are: a small pod type, with lengths between 30.0 and 80.0mm, and a big pod type with lengths of up to150.0mm., this last variety is used industrially and as fresh fruit, and the small pod type is used in juices and sweets.3 The information above, places the acid and the sweet tamarind in the big pod group and in the small pod group, respectively. This suggests that the acid phenotype has the potential for industrial usage and the sweet tamarind can be used in the market of diet products since additional sugars would not be necessary in preparations where the sweet tamarind is blended. Nevertheless, other characteristics should be evaluated to determine the real potential of each fruit type. The difference in the weight of the fruit could be due to the more attended management in the acid phenotype in aspects like pruning, weed control and fertilization. Even when these aspects are attended with low frequency, they are not practiced in sweet phenotype tamarind trees.
No. |
Descriptor |
Símbol |
Acid |
Sweet |
1 |
Pulp plus seed weight of 15 fruits (g) |
WPS |
99.4±19.2 |
82.1±18.5 |
2 |
Seeds weight of 15 fruits (g) |
WFS |
51.4±10.0 |
35.0±8.7 |
3 |
Pod length (mm) |
PL |
84.0±8.0 |
78.0±7.6 |
4 |
Seed length (mm) |
SL |
14.6±0.8 |
13.8±0.7 |
5 |
The red color component of the pulp |
Rpu |
44.2±6.0 |
37.1±5.5 |
6 |
The red color component of the epicarp |
Rep |
89.0±6.3 |
72.5±5.5 |
7 |
The green color component of the epicarp |
Gep |
66.1±5.8 |
54.9±5.0 |
8 |
The crown shape of the tree |
CS |
Pyramidal |
Oblong-Spherical |
9 |
Cortex texture |
CT |
Rough |
Brittle |
Table 4 Comparison of acid and sweet of the phenotype Tamarindus indica L, according to descriptors with statistical significance.
The tamarind color has been described as brown-cinnamon or brown-grayish.13 Evaluated individuals presented differences of the color of the epicarp, specifically in the red (R) and the green (G) components: Rep and GEp. The acid fruits had lighter brown-cinnamon shells (Rep=89.0 GEp=66.0). The shell of the sweet tamarinds was dark brown-grayish (Rep=72.6, GEp=54.9). This agreed with the local perception that the shell of the sweet tamarind is darker than the shell of the acid one. The pulp of the Sweet phenotype (RPu=37.0) was darker than the pulp of the acid one (RPu=44.0).
There was no significant difference among phenotypes in the sugar content of the pulp (TSS). The acid fruits did not differ with the sweet tamarinds in sugar content. An average of 45ºBrix was obtained, which is a high value compared to other fruits like sapodilla (Matisia cordata), that has 23ºBrix maximum values. When tasting, sapodilla it seems sweeter than the tamarind due to the high acid content in the tamarind, mainly the tartaric acid (8.0–23.8mg.100mg-1 of pulp) and ascorbic acid (0.7–3.0mg.100mg-1) that can mask sugars in the pulp. In addition, it is known that the reducing of sugars increases between 30% and 40% in the development of the fruit, while the high tartaric acid content does not decrease.2,13 On the contrary, the acidity percentages found were 4.52 and 2.55 for the acid and the sweet phenotype, respectively; i.e. the acid tamarind is 1.8 times more acid than the sweet tamarind, so the percentage of acidity was another differentiating factor between phenotypes. The Tamarind has been defined as a bittersweet fruit due to its high content of tartaric acids and sugars reducing combination, it is also said that it is the acidest and the sweetest fruit at the same time.2 The acid content of tamarind is a worthy feature because of its association with oil content and fatty acid content of its fruits and seeds.30 The acid and the sweet tamarind descriptors also differed in the crown shape (CS), the cortex texture (CT) and the seed shape (SS). 70% of the pyramidal shaped trees were acid and the 30% were sweet. The acid tamarind cortex texture was mostly rough (61%). On the other hand, the seed shape (SS) was not a differentiating characteristic between phenotypes.
The results could be related to the age of the trees because according to the farmers, the brittle cortex is presented in the longest-lived trees, which means that the most sampled sweet tamarind trees could have been planted before the acid tamarind trees. Similar to this work, a study carried out in Uganda found the relation between the land use type, wild or cutivatedplants, and the nutritional content of tamarind pods.30
Although morpho-agronomic characterization may be prone to subjective evaluation and cannot be directly correlated with the molecular data16 the phenotypical expression of plants is the basic unit of selection of local populations that depend of promissory species, that is why its study is important. However, complementary studies are recommended to be carried out with molecular characterization and so integrate with the knowledge of the communities, to have a complete vision of the intraspecific diversity.
The Two types of tamarind that exist in the tropical dry forest of Antioquia - Colombia, which are identified by the community as acid tamarind and sweet tamarind, are distinguishable from each other by their morpho-agronomic characteristics. The acid tamarind trees have pyramidal crowns and rough cortexes; with lighter colored epicarps and pulps; the pulps were also more acid than the sweet phenotype ones. The acid tamarind is the phenotype with more potential usage in the region, mainly its pulp exploitation in the elaboration of sweets. On the contrary, the sweet tamarind one have less productive characteristics than the acid ones have, this can be explained because of the lack of interest of the community in the exploitation and commercialization of the sweet tamarind fruit, or because of a genetic factor in this phenotype.
The 26 developed and evaluated descriptors of T. indica, allowed to group individuals with similar characteristics. Nine of these descriptors are useful to differentiate acid and sweet phenotype. The descriptors of weight of the fruit are important variables to study the diversity of the tamarind. A 32% of the diversity in the region means an important and promising value for the tamarind; this promotes studies to look for trees with potential fruit producing characteristics.
To The tamarind producers, harvesters and marketers of the nearby western of Antioquia (Colombia), to the Politécnico Colombiano Jaime Isaza Cadavid, funding institution of the research, to many friends, and to the research group of Tropical Agricultural Systems - SAT.
There is no conflict of interest to declare regarding the publication of this paper.

©2018 Osorio, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.