eISSN: 2575-906X


Research Article Volume 2 Issue 5
1Departamento de Producción Animal, Cátedra de Nutrición Animal, Facultad de Agronomía, Universidad de Buenos Aires, Argentina
2Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina
3Centro Austral de Investigaciones Científicas, Argentina
4Universidad Nacional de Tierra del Fuego, Argentina
5Laboratorio de Anatomía Vegetal, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Argentina
6Laboratorio de Paleobotánica, CICYTTP-CONICET, Argentina
Correspondence: Fernández Pepi, Departamento de Producción Animal, Cátedra de Nutrición Animal, Facultad de Agronomía, Universidad de Buenos Aires, Av San Martín 4453 (C1417DSE), Argentina
Received: August 10, 2018 | Published: September 19, 2018
Citation: Fernández PMG, Moretto AS, Arriaga MO, et al. Guanacos’ and domestic livestock’s summer diets comparison in ecotone of “Tierra del Fuego” (Argentina). Biodiversity Int J. 2018;2(5):425-431. DOI: 10.15406/bij.2018.02.00095
At present, it is believed that the population of guanacos has increased in the “Isla Grande de Tierra del Fuego”, arising a conflict with livestock and forestry activities. The aim of this study was to evaluate the use of the guanaco’s food resources, taking into account the presence of different types of domestic livestock, in order to provide a tool which will enable the evaluation of environmental management projects. We conducted the study in different areas, considering the presence/absence of domestic livestock and of guanaco. The diet was analyzed by identifying botanical remains present in the pretreated feces. The relative frequencies of ingested taxa were obtained and analyzed according to their functional groups, being the soft, herbaceous and graminoid grasses the most consumed, where the soft grasses were the most frequently ingested. Tree species only appear in diets of guanacos, in a low frequency, compared to other forms of life. In the case of soft grasses, forbs and Graminoides, the diets differ according to ingested species and intake frequency. These results allow us to establish that the guanaco selects the items to be consumed, changing its diet based on the presence of other herbivores, and has a trophic overlap with domestic livestock, mainly with sheep.
Keywords: herbivory, trophic overlap, lama guanicoe, vegetal microremains
The guanaco (Lama guanicoe) is the only ungulate that characterizes the native wildlife of Tierra del Fuego, Argentina. The last census, in the central zone, carried out in 2008, showed a density of 2.05km-2 individuals and a total of 14,000 individuals.1 The guanaco lives preferably in open grasslands,2 although it is also capable of living in forest areas, as it occurs in Tierra del Fuego,3,4 where it makes seasonal movements from the highlands in the summer towards the lowlands (seaside areas) which are free of snow throughout the winter and autumn.5 The guanaco is an opportunistic herbivorous, that survives in different habitats by making anatomic and physiologic adaptations.6
Additionally, in comparison to other ruminants, the guanaco has a great ability to digest low quality plants, what allows the guanaco to feed on a wide diversity of vegetation.7 Although the variations that were observed in the principal food items consumed by the wild populations of guanaco are dependent on the area’s features and on the vegetal species that are present in the different locations where the study was conducted, generally gramineous species were the plants which prevailed, followed in descending order by graminoids, bushes and herbaceous dicotyledonous plants, and in lower proportion by trees, lichen, epiphytes and cactuses.8‒16 The guanaco has the ability to alternate seasonally between grazing and browsing, according to available fodder, which allows to establish the guanaco behavior as a consumer of mixed adaptation who has the ability to digest low quality fodder.6,7,10 Its mouth structure enables the guanaco to select the part of the plants to consume, without uprooting them; facilitating the plants’ regrowth.17 Until the nineteenth century, the guanacos were present in almost all regions of Argentina, occupying different zones, from open habitats to scrublands and forests2 and from regions located on sea level to regions up to 4500 above mean sea level. Nowadays, guanacos are more numerous in the Patagonian steppe and in the borders of the Andes mountain range.2 This habitat shift has occurred as a consequence to the agricultural frontier expansion, that has taken over the original habitat of the guanaco. The province of Tierra del Fuego (Argentina) is not excluded from this situation. The guanaco food habits have been studied throughout the southern region of Argentina, in the steppe, the foothills and the mountain range of the Andes and in the ecotonal zones of the Patagonia region.3,8,10,14,18,19 However, the population dynamics and the habitat use of the guanacos is yet scarcely known. The herbivores, on the basis of their selectivity and preference, directly affect certain species, while acting indirectly on others by exerting an influence on the habitat in which they are found. It is therefore important to know the diet of grazing animals, determine which species are highly consumed, the variability in composition according to availability, the season of use of different plant species, the degree of overlap of diets of different kinds of animals. Knowledge of nutritional habits in ecosystems is important for the study and interpretation of the flow of energy through them, and also allows to establish the influence of each herbivore on the composition and physiology of the community.20
According to previous work in the area of the fuegian ecotone, some degree of modification in the composition of the vegetation can be observed, one of the possible causes being the disturbance caused by overgrazing, which has led to a change in the botanical composition in it, leading to a decrease in the coverage of native species and advances in invasive species, such as Hieracium pistosella.21,22 This has also been observed in the steppe fuegian, in response to the introduction of sheep.23 This is why it is essential to develop practical management standards to avoid further degradation of this type of ecosystem and increase its productivity.
The technique of recognition of micro-remains plant is universal, allows the study of nutritional habits through the analysis of ruminal, stomach, esophageal or stool content, which is why this type of research in herbivores is facilitated. The application of the microhistological analysis technique, together with the study and recognition of the species of components of the animal diet, constitute an important step for obtaining management plans for natural areas.14 Our purpose was to quantify the diet of the guanaco and of other domestic animals, taking into account the vegetal composition of the areas where these animals are present, so as to provide a tool to evaluate projects of sustainable handling and of environment preservation in ecotone fuegian.
Study area and sampling locations
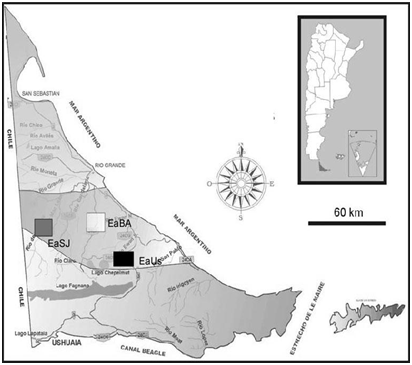
The study was conducted in the ecotone area of the province of Tierra del Fuego (Argentina). This area, located between the Magellanic steppe and the southern wooded area, covers the central zone of the island. The locations were selected according to the presence of herbivores A) Ranch “Estancia. Buenos Aires” (EaBA) features grasslands where the species Festuca magellanica and Poa pratensis prevail and that are associated to flood meadows with predominance of herbaceous >dicotyledons, such as Caltha sagittata, where sheep and guanacos overgraze; B) Ranch “Estancia Ushuaia” (EaUs) presents meadows of gramineous grasses, Cyperaceae, and cushion species, such as Azorella trifulcata, and on the mountainside there are forests of ñire (Nothofagus antarctica) and of lenga (N. pumilio) and also cows, horses and guanacos are found; C) Ranch “Estancia San José (EaSJ), which has grasslands where gramineous grasses and Cyperaceae are dominant, has been overgrazed uniquely by guanacos for the last 10 years (Figure 1).
Vegetation sampling and analysis
To determine the floral and diets composition, plants of the present communities, and feces belonging to guanacos, horses, cows and sheep were collected during the summer of 2010. A list of the vegetal species present in the area was drawn up.24‒26 These species were then classified according to their life form into: Cushion (Cu), Creeping bushes (CrBush), Erect bushes (ErBush), Grasses (G), Herbaceous dycotiledons (HD), Tree species (Tr), Graminoids (Gr), Moss/Lichen and Bracken (MLB) (Table 1).23 The collected samples were used as reference material (sensu 24). In addition, vegetational censuses were carried out in each community by use of the quadrant method27 where 160cm2 squares (40x40cm) are established. We settled ten transects over each community, making 10 measurements per transect. The data were registered as percentage values, according to each functional group. For practical purposes, the ranges measured in field were averaged.
The composition of the communities was analyzed by the nonparametric Kruskal-Wallis test, and to make a comparison between the floral communities, the Sörensen Index (SI) was applied:28 IS=(2xC)/(Gi+Si), where: C represents the number of common species between both communities; Gi the number of species that are present in community A; and Si the number of species that are present in community B. SI values above 0,75 are considered to reflect a very high similarity; SI values from 0.51 to 0.75 show a high similarity; and values between 0.26 a 0.50 indicate a moderate similarity. Low similarity values correspond to those below 0.25.29
Funcional group |
Ushuaia ranch (EaUs) |
San josé ranch (EaSJ) |
Buenos aires ranch (EaBA) |
Cushion (Cu) |
Azorella trifurcata |
Azorella filamentosa |
Azorella filamentosa |
Bolax gummifera |
Azorella trifurcata |
|
|
|
Bolax gummifera |
|
|
Grasses (G) |
Agrostis perennans |
Agrostis perennans |
Alopecurus magellanicus |
Alopecurus magellanicus |
Alopecurus magellanicus |
Bromus coloratus |
|
Bromus coloratus |
Bromus coloratus |
Deschampsia patula |
|
Deschampsia patula |
Deyeuxia poaeoides |
Deyeuxia poaeoides |
|
Elymus sp. |
Elymus sp. |
Elymus sp. |
|
Festuca magellanica |
Elytrigia repens |
Elytrigia repens |
|
Elytrigia repens |
Festuca magellanica |
Festuca magellanica |
|
Hordeum pubiflorum |
Hordeum pubiflorum |
Festuca monticola. |
|
Koeleria fueguiana |
Koeleria fueguiana |
Hordeum pubiflorum |
|
Phleum alpinum |
Phleum alpinum |
Koeleria fueguiana |
|
Poa pratensis |
Poa pratensis |
Phleum alpinum |
|
Trisetum spicatum |
Trisetum spicatum |
Poa pratensis |
|
Graminoids (Gr) |
Luzula alopecurus |
Luzula alopecurus |
Luzula alopecurus |
Carex macloviana |
Carex macloviana |
Carex macloviana |
|
Uncinia sp. |
|
Juncus sp. |
|
Herbaceous dycotiledons (HD) |
Acaena magellanica |
Acaena magellanica |
Acaena magellanica |
Anemone multifida |
Caltha sagittata |
Anemone multifida |
|
Colobanthus sp. |
Cerastium arvense |
Armeria maritima |
|
Draba magellanica |
Erygeron myosotis |
Caltha sagittata |
|
Erodium cicutarium |
Euphrasia Antarctica |
Cerastium arvense |
|
Erygeron myosotis |
Galium aperine |
Colobantus sp. |
|
Galium aperine |
Gentinella magellanica |
Draba magellanica |
|
Gentinella magellanica |
Gunnera magellanica |
Erodium cicutarium |
|
Geranium sp. |
Hieracium pillosela |
Euphrasia Antarctica |
|
Gunnera magellanica |
Leptinella scariosa |
Galium aperine |
|
Hieracium pillosela |
Myosotis arvensis |
Gentinella magellanica |
|
Leptinella scariosa |
Nassauvia darwinii |
Geranium sp. |
|
Myosotis arvensis |
Osmorhiza chilensis |
Gunnera magellanica |
|
Nassauvia darwinii |
Oxalis enneaphylla |
Hieracium pillosela |
|
Osmorhiza chilensis |
Perezia pilifera |
Leptinella scariosa |
|
Oxalis enneaphylla |
Rumex acetosella L. |
Myosotis arvensis |
|
Perezia pilifera |
Senecio magellanico |
Nassauvia darwinii |
|
Phacelia secunda |
Veronica |
Perezia pilifera |
|
Primula magellanica |
Vicia |
Phacelia secunda |
|
Ranunculus penduncularis |
Taraxacum officinale |
Primula magellanica |
|
Rumex acetosella |
Trifolium repens |
Rumex acetosella |
|
Senecio magellanico |
|
Stellaria sp. |
|
Stellaria sp. |
|
Silene sp. |
|
Silene sp. |
|
Taraxacum officinale |
|
Veronica sp. |
|
Trifolium repens |
|
Taraxacum officinale |
|
|
|
Creeping bushes (CrBush) |
Berberis microphylla |
|
Berberis microphylla |
Berberis empetrifolia |
|
|
|
Erect bushes (ErBush) |
Chiliotrichum diffusum |
Chiliotrichum diffusum |
Empetrum rubrum |
Empetrum rubrum |
Empetrum rubrum |
Gaultheria sp. |
|
Gaultheria sp. |
Gaultheria sp. |
Chiliotrichum diffusum |
|
Tree species (Tr) |
Nothofagus antarctica |
|
|
Nothofagus pumilio |
|
|
Table 1 The list of plant species is presented in the study area. The classification into functional groups is presented according to their form of life
Herbivore feces sampling and analysis
Food habits of herbivores were studied during summer 2010 by randomly collecting 1-5 to dung units of every dropping at each feeding area. Faeces samples were spread over paper and dried at ambient temperature for several days and then put into paper bags. Later on, the grounded samples were boiled with 5% NaOH for 1-2 minutes and then rinsed with NaClO, bleached for a few minutes, and thoroughly rinsed in water again. Three slides were made from each sample for microscopic observation, 9 preparations were made per stay, for each station sampled, and 20 optical fields were quantified for each one.
Diet analysis
The diet was analyzed by identifying the presence of botanical remains in the feces, that were previously handled according to the method proposed by Arriaga.30 The species’ relative frequencies were obtained and analyzed according to their life form by using the nonparametric Kruskal-Wallis test. The diversity index (H)31,32 was used to compare the diversity among herbivorous species. The diversity diet was carried out with the T test. To analyze the general variation trends throughout and between the communities in the proportion of consumed species by the herbivorous animals, an analysis of the main components was carried out. All analyses were performed with the PAST programme.33
When comparing the floral composition of the three vegetal communities measured in the ranches, by use of the SI, a great similarity regarding the vegetal species was found at the ranches, given that all the calculated indexes show values between 0.51 and 0.75 (Table 2).
|
EaBA |
EaSJ |
EaUs |
0.74 |
0.73 |
EaSJ |
0.73 |
----- |
Table 2 Comparison of the vegetal composition of the three studied communities, using the Sörensen Index. Ranch “Estancia Buenos Aires” (EaBA), Ranch “Estancia San José “(EaSJ) and Ranch “Estancia Ushuaia” (EaUs)
Regarding the diet composition diversity, among the calculated H indexes, the one corresponding to sheep from EaBa show significant differences compared to the other H indexes that were calculated for the rest of the herbivores included in this study (p<0.05), being the sheep diet the most diverse (Table 3). Guanaco´s H indexes, calculated in all studied areas, was not express any significant interrelated differences (p>0.05) (Table 3), what would indicate that the guanaco ingests the same species quantity in all communities. The lowest H indexes corresponded to cow and horse diets (Table 3), being the least varied when analyzing their composition.
|
H |
EaBAG Guanaco |
2.73 |
EaBAO Sheep |
3.04 |
EaUG Guanaco |
2.79 |
EaUC Horse |
2.45 |
EaUV Cow |
2.29 |
EaSJG Guanaco |
2.75 |
Table 3 Diversity index (H) calculated for the diets of each of the herbivores studied. Ranch “Estancia Buenos Aires” (EaBA), Ranch “Estancia San José “(EaSJ) and Ranch “Estancia Ushuaia” (EaUs)
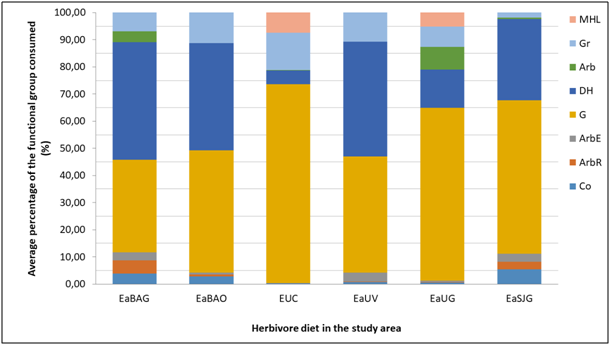
The most consumed life forms are, in decreasing order, G, HD and Gr (Figure 2). In the case of EaBa, the intake frequency of the sheep presented no significant differences compared to the guanaco’s, whereas in EaUs considerable differences are found between the horse and the other two studied herbivorous animals (p0.05) (Figure 2). The guanaco expressed major differences in its diet composition when comparing the sampling locations (p0.05) and taking into account all life forms (Figure 2). SG were consumed more frequently in domestic cattle absence, whereas when domestic cattle was presented, the ingest of this lifeform decreases and the intake of HD and Gr increases (Figure 2). This agrees with what was before described about the guanaco diet in Tierra del Fuego,8,16,19,29,31,32 in arid and semi-arid areas of the Patagonia.34‒38
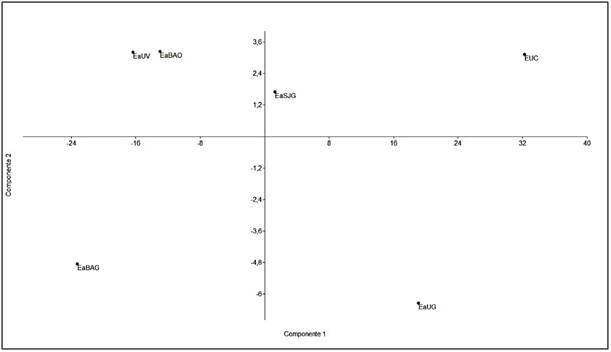
The main consumed species by all herbivores were Poa pratensis, Elytrigia repens, Acaena magellanica, Gunnera magellanica, Carex macloviana and Luzula alopecurus. Intake was observed, with low frequency, in all diets the presence of an invasive species, Hieracium pistosella, which is being studied for its degree of progress in Tierra del Fuego.23,39,40 When comparing the diet composition to the main components analysis, according to components 1 and 2 (97% of total variability), it was observed that cows and sheep group by the HD intake frequency (Figure 3), whereas guanacos differ according to the sampling area. The guanaco diet in the EaSJ was different from the others because of the intake frequency of Gr and SG; whereas in EaBa the diet was different due to the presence of bush species; and in EaU because of the intake of MLB (Figure 3). Previous works have provided information about the trophic overlap that occurs between guanacos and other domestic herbivorous animals, mainly cows and sheep14,18,34,35,41,42 and about the change in selected items by the native herbivore before the different situations. The guanaco, as a generalist herbivore of intermediate selection, is capable of consuming most available species of plants, from grasses to ligneous species, but mainly bushes.14,36,43,44 Indirect evidence suggests that prairies are the preferred habitat for guanacos in Tierra del Fuego, but that they use forest patches due to displacement by sheep.3 In a continental site where the guanacos were sedentary, it was shown that the ewes excluded guanacos from the prairies through resource competition.42 The effect of the presence of the sheep on the guanaco density and habitat use and selection have not been studied quantitatively in the grassland-forest mosaic of Tierra del Fuego.44 Domestic sheep, the main animals introduced for livestock purposes in the distribution range of the guanaco in Tierra del Fuego, are also generalists of intermediate selection, and present a greater trophic overlap with the guanaco in this study. This result agrees with the one obtained by Puig et al.45 for other Patagonia areas. The trophic overlap degree between the guanaco and domestic cattle (sheep, cow and horse), regarding consumed species and intake frequency, is similar to the results found in the studies previously carried out by Puig et al.,45 Fernández Pepi et al.,14 and Linares et al.15
The guanaco’s dietary flexibility and its condition as an intermediate consumer enables them to adapt efficiently to seasonal changes and to minimize the food competition with other herbivorous species, mainly during times of shortage.15 Since guanacos migrate seasonally between forests and meadows,46 as a combined effect of habitat requirements and overlap with domestic animals, the data obtained here complements previous studies in the area to gain knowledge in the ecology of the guanaco and the possible consequences of the dietary changes and use of habitat for the conservation and management of the species, as stated in theirs works Martínez Pasteur et al.,47 and Flores et al.48
This work contributes to enhance the knowledge about the guanaco diet, taking into consideration life forms, vegetal species and the intake frequency, according to the domestic livestock and to the present vegetal availability in each studied ranch. This broadens and updates the information about the diet and the use of resources of the guanaco, a native herbivorous animal in the ecotone of Tierra del Fuego, in function of the domestic livestock (sheep, cow and horse), and contributes new data on the comparison between the guanaco and the horse. This kind of comparative study is important in order to evaluate possible trophic overlaps, changes in the vegetal communities’ biodiversity, constituting a helpful tool that may be used in projects of sustainable management of resources and environments. This would allow to elaborate management norms in the grazing systems taking into account the key forage species and the advance of invasive species, than Hieracium pistosella, adjust the animal load of the domestic livestock in adequate proportions, increase quantitatively the availability of the natural resources and avoid the exhaustion thereof.49‒51
This work was carried out as part of the “Bases Project for the sustainable use of the guanaco in Tierra del Fuego” (2007-2010). Federal Projects of Productive Innovation (“Proyectos Federales de Innovación Productiva”). We are grateful to the owners of EaBa, EaSJ and EaUs for their kindness during the samplings. To Miss Ma. Dolores Montero for her contributions in the handling of the herbarium material. In addition, we would like to thank the reviewers for their valuable contributions to improve the manuscript.
The author declares that there is no conflict of interest.

©2018 Fernández, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.