eISSN: 2575-906X


Research Article Volume 3 Issue 6
1Grupo de Investigación en Zoología, Facultad de Ciencias, Universidad del Tolima, Barrio Santa Helena parte alta, Ibagué, Tolima, Colombia
2Department of Wildlife and Fisheries Sciences, and Biodiversity Research and Teaching Collections, Texas A&M University, USA
3Department of Ecology, Universidad Autónoma de Madrid, Spain
Correspondence: Diana Carolina Montoya Ospina, Grupo de Investigación en Zoología, Facultad de Ciencias, Universidad del Tolima, Barrio Santa Helena parte alta, Ibagué, Tolima, Colombia, Tel (+57) 312 312 2878
Received: November 06, 2019 | Published: November 26, 2019
Citation: Ospina DCM, Delgado EOL, Hevia V, et al. Effects of habitat structural complexity on diversity patterns of neotropical fish assemblages in the Bita river basin, Colombia. Biodiversity Int J. 2019;3(6):267-274. DOI: 10.15406/bij.2019.03.00154
Several studies have shown that fish assemblages are structured by habitat features, most of them have proposed that there is a positive relationship between habitat structural complexity and species diversity. In this study, we aimed to test this positive-relationship idea in three habitats (creeks, oxbow lakes and river sandbanks) distributed along the Bita River Basin Colombia, South America. Standardized surveys were conducted during January and February of 2016 (low water period) at 30 sites distributed along the entire basin. We recorded 23,092 individuals representing 191 species of fish. To investigate possible relationships between habitat structural complexity and species diversity, we calculated the first three Hill’s numbers, and performed a Non-metric Multidimensional Scaling (NMDS), a Principal Component Analysis (PCA) and a Canonical Correspondence Analysis (CCA). Our results showed that river sandbanks and creeks harbored the highest species richness. Results from the NMDS analysis (stress=0.19) showed that fish community composition was different in the assessed habitats (ANOSIM < p=0.001). According to the results of the principal component analysis, sand percentage, dissolved oxygen, and vegetation width separated the river sandbanks from the other habitats. Results from the Hill’s numbers, forward selection procedure, and canonical correspondence analysis suggested that species composition and diversity were significantly influenced by the habitat structural complexity index and conductivity.
Keywords: biodiversity; structural complexity; Orinoco River Basin; Colombia.
The Neotropical region is the most biodiverse area in the world. Regarding fish species, in this region has been described more than 6,000 species and the number increases by day.1 In the South American part of the Neotropical area, the Amazonas and Orinoco river basins are the largest and most biodiverse. The Orinoco River Basin extends to Colombia and Venezuela harboring roughly 1,000 fish species,2 just in the Colombian part, there are more than 663 species which represents 45% of the Colombian fish diversity.3
Given the current crisis of biodiversity loss due to anthropogenic impacts,4 it is crucial to study diversity patterns in these mega diverse regions. In the Orinoco river basin, the major threats are deforestation, land use change, damming, mining and overfishing.2 These affect biodiversity in different ways. For instance, Montag found that land use change is an important driver of biodiversity in Neotropical freshwater systems. Another important driver of biodiversity is habitat heterogeneity, in a Neotropical river of South America Willis et al.,6 found that fish assemblages were positive associated with habitat structural complexity. They found that species richness and evenness in structural complex habitats were related with niche partitioning.
The influence of habitat heterogeneity on fish species composition is a well-known phenomenon.5 Habitat heterogeneity is related to different ecological processes such as species richness, abundance, competition and many more.6 Some studies have found that high spatial heterogeneity often results in different microhabitats which increase biotic diversity.7,8
Habitat structural complexity influences fish assemblages in many ways. Several freshwater studies have found significant relationships between species richness and habitat complexity.6,9–11 Loss of habitat complexity affects diversity and distribution of fish assemblages by decreasing the diversity of native species, which results in biotic homogenization of fish communities.2,12–20
Previous ichthyological research in the Bita River basin aimed to describe and characterize fish communities.21,22 Recently López-Delgado et al.,11 evaluated fish metacommunity in the Bita River along the longitudinal gradient and found that species sorting and environmental filtering were the principal structuring forces in this system. Our study aimed to identify the effects of habitat structural complexity on the richness and diversity patterns of fish communities in the Bita River drainage (Vichada, Colombia). We proposed that habitat structural complexity is related to fish richness and diversity, and hypothesized that as habitat structural complexity increases, fish diversity will increase too.
Study area
The Bita River drainage is located in the eastern plains of the Colombian Llanos in the Vichada department (Colombia), draining directly to the Orinoco River, with a water course distance of 700km approximately, and a total area of 812,312 ha. This region is characterized by an unimodal pluviometric regimen.23 The main river and streams contain multiple habitats that vary mainly in substrate composition, riparian vegetation and depth, average air temperature in the region is 27°C, and average annual precipitation is 2,300 mm with most rainfall from May to August.11,22
Sampling
The sampling campaign was carried out during the low water period (January, February 2016, dry season) when sampling efficiency is higher and the relationship between fish species and habitat should be higher. Standardized surveys were performed using a seine net (10m x 2m; 3 mm mesh) and three gillnets (30m x 2m; 5cm mesh) at 30 sites distributed along the entire Bita River basin. Sampling sites were distributed in 11 river sandbanks, 10 creeks locally named “caños” and nine oxbow lakes “madreviejas” (Figure 1, Supplementary material Table 1). At each site we conducted six seine hauls in a 200 m reach and deployed the gillnets for 2 hours, checking them every hour.
|
Variables |
Factor 1 |
Factor 2 |
|
|
Water parameters |
pH |
0.12103 |
-0.2879 |
|
Water temperature (°C) |
0.02394 |
-0.9045 |
|
|
Electrical conductivity (μS cm–1) |
-0.36653 |
-0.7472 |
|
|
Dissolved oxygen (mgO2 L–1) |
0.53332 |
1.6E-05 |
|
|
Instream cover variables |
%Sand |
0.76481 |
0.1369 |
|
%Mud |
-1.02215 |
-0.0876 |
|
|
%Cobble |
0.22983 |
0.2619 |
|
|
%Algae |
-0.30023 |
0.2476 |
|
|
% Big trunks |
-0.83098 |
-0.0358 |
|
|
% Small trunks |
-0.90249 |
0.1392 |
|
|
%Roots |
-0.79517 |
-0.1787 |
|
|
%Leaf litter |
-0.37085 |
-0.3266 |
|
|
% Riparian vegetation width (m) |
-1.03458 |
0.9301 |
|
|
%riparian vegetation cover |
-0.07621 |
0.2822 |
|
|
Forest cover |
-0.64825 |
-0.2923 |
|
|
Channel morphology |
Width |
0.70905 |
-0.5521 |
Table 1 Contribution of environmental variables to the first three factors for habitats in the Bita river basin
After catch, fishes were removed from the nets, anesthetized and euthanized by immersion in tricaine methane sulfonate (MS-222), then fixed in 10% formalin. In the laboratory, they were transferred to 70% ethanol for final preservation and were identified using specialized taxonomic literature.24–48 Finally, specimens were deposited in the ichthyological collections of the Universidad del Tolima, Colombia (CZUT-IC) and Instituto Alexander von Humboldt, Colombia (IAvH-P).
Prior to the surveys, at each sampling site we measured environmental variables following the methodology proposed by Pease et al.9 Variables were divided into four categories: water parameters, channel morphology, instream cover, and riparian cover. Water parameters such as pH, conductivity, dissolved oxygen and water temperature were measured using a multiparameter meter (YSI model 85). Channel width (m), width of the riparian vegetation, and depth (superficial <1m, moderate between 1-3m, deep > 3m), were measured using a metric tape, and the water flow was visually categorized as high, medium, and low. To characterize instream cover we visually estimated the percentage of mud (<0.06mm), sand (0.06–2mm), pebbles (6–25cm), filamentous algae, large trunks, small trunks, branches, roots, leaf litter, and riparian vegetation cover along the 200 m reach (Table 1).
Structure and composition
We calculated the relative abundance using the number of specimens collected from each species divided by the total number of specimens in the sample. Fish diversity was calculated using the first three Hill’s numbers q= 0 (species richness), q=1 (the exponential of Shannon’s index), and q=2 (the inverse of Simpson’s index) following the equation proposed by Jost49
Where qD is the diversity. The exponent q determines the sensitivity of the index to the relative abundances of the species.50 Additionally, to identify if diversity was different among the habitats, we applied the bootstrap method to construct confidence intervals around the interpolated and extrapolated Hill’s numbers as proposed by Chao et al.,51 using the R library iNEXT.52
To establish if community structure was similar in each habitat, a Non-metric Multidimensional Scaling analysis (NMDS) was performed, using the Bray-Curtis distance. NMDS was considered robust when the stress value was less than 0.2. Additionally, we performed a non-parametric Similarity Analysis (ANOSIM) 53 to determine if there were statistically significant differences between groups (habitats). Statistical analyses were carried out using the R library Vegan and the functions metaMDS and anosim.
Habitat complexity
Habitat complexity was estimated using the habitat structural complexity index (S) proposed by Winemiller et al.54
Where S is the structural complexity index, xi represents each component of the instream cover in each sampling site, and N is the number of joint components of the instream cover observed at the sampling sites. Values close to zero indicate low complexity and close to one, high complexity.
Environmental factors
To reduce the dimensionality of the environmental variables and to identify if the habitats were separated based on instream cover and habitat features, we performed a Principal Components Analysis (PCA) on the correlation matrix with the data previously log(x+1) transformed.
The components retained for interpretation were chosen according to the broken stick model55 in which a pattern of expected eigenvalues is estimated and only those components that exceed these values will be considered significant. These analyses were performed using the PCA function from the R library “FactoMineR” and the PCA significance function from the R package “BiodiversityR”.52
To determine if environmental variables were related to fish community structure, we performed a Canonical Correspondence Analysis (CCA) followed by a forward selection procedure with 999 permutations and a significance level of p=0.05. To perform the CCA, we used the Hellinger-transformed species matrix and the environmental variables log(x+1) transformed. For the environmental matrix we removed all the instream cover variables because they were used to calculated the structural complexity index, which could result in multicollinearity that could affect the performance of the analyses. These were conducted using the function cca from the “vegan” library and the function forward.sel from the R library “adespatial”.
Structure and composition
We collected 23,092 specimens distributed in 10 orders, 39 families, 125 genera and 191 species. Characiforms was the most abundant order (79.34%), followed by Clupeiformes (9.99%) and Cichliformes (5.24%), while the others had an abundance lower than 4%. Characidae was the most abundant family (70.39%), and the most abundant species were Hemigrammus elegans (12.38%), Hemigrammus geisleri (11.46%), Amazonsprattus scintilla (9.50%), Hyphessobrycon diancistrus (7.26%), Hemigrammus analis (6.14%), Microschemobrycon casiquiare (4.62%) and Hemigrammus newboldi (4.31%).
Regarding habitats, 47.67% (10,085) of the individuals were collected in the river sandbanks belonging to nine orders, 33 families, 80 genera and 122 species. The most abundant species were H. geisleri (25.9%), A. scintilla (20.5%), M. casiquiare (9.1%) and Knodus cinarucoense (7.83%). In the creeks, 29.91% (6909) of the individuals were collected, belonging to nine orders, 33 families, 90 genera, and 122 species. The most abundant species were H. elegans (27.6%), H. newboldi (9.36%), H. analis (8.16%) and Tyttobrycon xeruini. (7.5%). Finally, in the oxbow lakes we collected 26.40% (6098) of the total number of individuals, belonging to 9 orders, 33 families, 78 genera, and 108 species. The most abundant species were Hyphessobrycon diancistrus (24.65%), H. elegans (15.02%) and H. analis (8.23%) (Table 2).
With respect to the effective number of species, results from the order q=0 showed that the river sandbanks (122 spp.) and creeks (122 spp.) had the greatest species richness and the oxbow lakes the lower (Figure 2). However, when checking the confidence intervals, we did not observe significant differences among habitats. In contrast, orders q=1 and q=2 showed significant differences between the evaluated habitats (Figure 2). The order q=1 and q=2 showed that creeks and oxbow lakes obtained the highest effective number of species, and the lowest were observed in the river sandbanks (Figure 2).
|
Variables |
|
Creek |
Oxbow lake |
River’s sandbanks |
|
pH |
7.92±1.25 |
7.2±0.75 |
8.03±1.30 |
|
|
Water temperature (°C) |
28.9±1.39 |
29.63±1.72 |
29.1±1.25 |
|
|
Water parameters |
Electrical conductivity (μS cm–1) |
5.85±2.84 |
44.47±48.97 |
5.02±1.25 |
|
Dissolved oxygen (mgO2 L–1) |
9.06±2.44 |
8.49±1.54 |
10.38±1.41 |
|
|
%Pebble |
0 |
0 |
14.55±32.67 |
|
|
%Sand |
15.5±21.92 |
28.89±31.4 |
85.45±32.57 |
|
|
%Mud |
77±29.83 |
50±32.02 |
0 |
|
|
%Algae |
5±9.72 |
1.11±3.33 |
0.91±3.02 |
|
|
Instream cover variables |
%Large trunks |
11±8.76 |
11.11±10.54 |
3.64±8.09 |
|
% Small trunks |
25±10.8 |
16.67±6.61 |
4.55±6.88 |
|
|
%Roots |
7±9.49 |
7.78±7.12 |
2.73±9.05 |
|
|
%Leaf litter |
48±17.51 |
47.22±16.41 |
8.64±5.52 |
|
|
% Riparian vegetation width (m) |
6±6.99 |
10±11.46 |
0.91±3.02 |
|
|
Riparian vegetation |
20±29.81 |
8.11±9.13 |
14±16.56 |
|
|
%Riparian vegetation cover |
50±24.61 |
21.67±13.69 |
2.27±4.10 |
|
|
Structural complexity (S) |
0.65±0.12 |
0.63±0.16 |
0.31±0.17 |
|
|
Width |
13.4±9.19 |
61.11±90.41 |
73.64±55.19 |
|
|
Channel morphology |
Depth |
2.5±0.53 |
2.11±0.78 |
2.36±0.5 |
|
|
Water flow |
1.1±0.32 |
1±0 |
1.91±0.70 |
Table 2 Physical, physicochemical and structural Variables of the habitats of the Bita river basin. Mean ± standard error

Figure 2 Extrapolation and interpolation of the effective numbers of species of the orders q=0, q=1 and q=2 in the habitats of the Bita river basin (Vichada, Colombia).
Results of the NMDS analysis (stress=0.19) showed that fish community composition differed in all the assessed habitats (ANOSIM, P <0.001) (Figure 3). Creeks and oxbow lakes were close to each other in the ordination diagram, because they share about 70% of the species. River sandbanks were the most isolated habitat in the ordination space, due to its particular composition, characterized by species such as: Serrasalmus irritans, Bivibranchia fowleri, Bivibranchia velox, Boulengerella lateristriga, Brycon pesu, Acestrorhynchus falcatus, Loncogenys ilisha, Acestrocephalus sardine, Astyanax bimaculatus, Creagrutus phasma, Microschemobrycon callops, Knodus cinarucoense, Roeboides affiis, Leptodoras linnelli, Phenacogaster prolatus, Mastiglanis asopos, Haemomaster venezuelae, Eigenmannia macrops, Cichla temensis, Panaqolus maccus and Paratocinclus eppleyi.
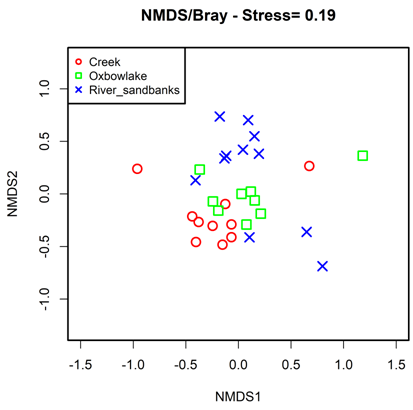
Figure 3 Non-metric multidimensional scaling, of the fish fauna composition in the habitats evaluated along the Bita river basin.
Habitat complexity
The S index showed that the sampling sites located in the creeks, and oxbow lakes had the highest values of habitat structural complexity. On the contrary, sampling sites located at the river sandbanks (E7, E20, E30, and E33) had the lowest values (Table 2).
Environmental factors
Using the broken stick model, we selected the first two components of the PCA. These two modeled 44.5% of the total variation (Figure 1). The first axis (modeling 30.1% variation) was positively associated with percentage of sand, width, and dissolved oxygen, and negatively with riparian cover and instream cover variables such as; percentage of leafpacks, mud, roots, and small and large woody debris (Table 1) separating creeks and oxbow lakes from river sandbanks (Figure 4). The second axis (modeling 14.4% variation) was negatively associated with water temperature and conductivity and positively with vegetation wide (Table 1). This axis described a physical gradient that separated some oxbow lakes, river sandbanks and creeks (Figure 4, Table 1).
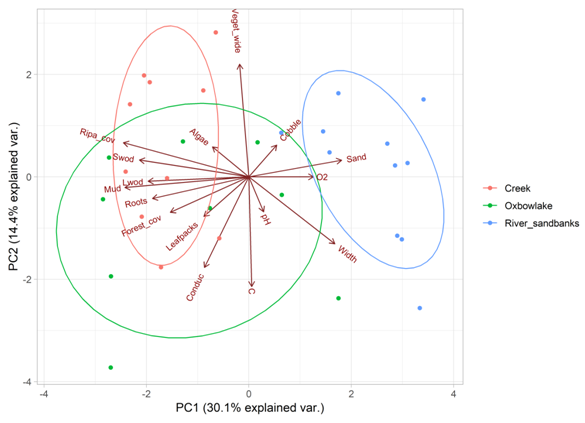
Figure 4 Principal Component analysis (PCA) of the habitats evaluated in the Bita river basin (Vichada, Colombia), based on habitat and environmental variables.
In general, environmental variables explained 34% of species variation in the assessed habitats. Species such as: Leporinus fasciatus, Hemigrammus elegans, Batrochoglanis villosus, Dicrossus filamentosus and Curimatopsis evelynae, were associated to creeks and oxbow lakes. These species were also related to the structural complexity index, conductivity and forest cover (Figure 5). In river sandbanks, we found species such as: Leporinus friederichi, Limatulichthys griseus, Knodus cinarucoensis, Moenkhausia copei, Amozonpratus scintilla, Aphyochrax alburnus, Bivibranchia fowleri, Creagrutus phasma and Eigenmannia macrops, which were associated with dissolved oxygen and river width (Figure 4). According to the results of the forward selection procedure, only structural complexity (p= <0.001) and conductivity (p= 0.02) were significantly correlated with species distribution. Ordination diagrams (Figures 4&5) showed that creeks and oxbow lakes were similar in terms of species composition and environmental variables. These two habitats shared many species that inhabit sites with high structural complexity and forest cover.
In this study, we investigated if there was a positive relationship between habitat structural complexity and fish diversity in a Neotropical river basin. To do that, we evaluated three habitat types (creeks, oxbow lakes and river sandbanks) along the longitudinal gradient of the Bita river basin as a mean to compare habitats with different structural complexity. Our results suggest that environmental variables such as habitat structural, complexity index and conductivity were significantly associated with species abundance and distribution. In general, it was observed that as the structural complexity index increased fish diversity increased too, supporting in part our initial prediction. We expected that survey sites with low habitat structural complexity index will show low species richness. However, in river sandbanks were the values of structural complexity index were low we found a high species richness.
Results of the first three orders of Hill’s numbers revealed different diversity patterns. According to the order q=0 which measures species richness without taking in to account abundance, we did not find significant difference among the evaluated habitats, rejecting our working hypothesis. However, when evaluating the others two orders which take into account rare and common species, river sandbanks registered low values of effective number of species supporting our hypothesis. These results showed the importance of using diversity measures that go beyond the number of species. As proposed by Chao et al.,51 it is crucial to consider evenness and inequality when studying biodiversity.
The highest values of the habitat structural complexity index were registered in creeks and oxbow lakes, these habitats were characterized by higher percentages of tree branches, trunks, roots, leaves and lower water flow. The high diversity and richness of these habitats could be related to the fact that the trunks and woody debris contribute to habitat diversification,56 favoring the formation of microhabitats by increasing the spatial heterogeneity at the bottom of the river.57,58 For instance, species such as Tatia marthae, Tatia nigra, Batrochoglanis villosus, Ochmacanthus alternus, Amblydoras bolivarensis, Acanthodoras cataphractus among others, inhabit inside trunks for protection and foraging on invertebrates.6 In addition, these structures provide surfaces that favor the presence of invertebrates that are a food source of other fish species,59 and refuge for different life stages of fish fauna.14 Studies in the Cinaruco River (Venezuela), a similar system to ours have reported that structural complexity in creeks and oxbow lakes is strongly related to fish diversity, where the niche packing and habitat segregation seem to be responsible for the high diversity.6
Riparian cover was one of the variables that separated creeks and oxbow lakes from river sandbanks, studies in the Neotropics have shown that a better vegetal cover is related to a better water quality, that increase the number of native species and decrease the number of dominant and invasive species.60 The vegetal cover is also important because it is a source of allochthonous material, which is used as food and shelter for aquatic species.14,16,59–61 Leaflitter, roots and submerged branches support a higher primary and secondary productivity providing more foraging sites with diverse substrates for fish species.6 This could explain the presence of invertivores species such as Copella nattereri, Anostomus ternetzi, Acaronia vultuosa and Geophagus abalios among others.
Submerged vegetation produces an increase in shade, lower water temperature, and different types of microhabitats that fish species can exploit.62 This habitat feature traps detritus in its leaves and roots,63 which is used by detritivores species such as Cyphocharax spilurus. Additionally, submerged vegetation provides camouflage and refuge to some species (Dicrossus filamentosus, Elachocharax pulcher, Apistogramma honsloi, physopysix lyra) to hide from predators such as carnivorous species (Hoplerythrinus unitaeniatus, Crenicichla wallacii, Acestrorhyncus microlepis), and it is used for some species as spawning areas.64,65
In southeastern Brazil, Gerhard et al.,66 identified patterns of association between fish communities and habitat variables. Their results suggested that species exchange in the lower parts of rivers is due to an increase in the ecosystem productivity and greater habitat complexity. Our results suggested that fish assembly in the different habitats of the Bita River drainage respond to habitat structural complexity, as well as to the influence of conductivity.
Contrary to our prediction and contradicting most of the literature on fish diversity and habitat structural complexity, we registered a high species richness and the greatest abundance in river sandbanks. This habitat registered the lower values of the structural complexity index and was characterized by a homogeneous sand substrate, greater channel width, and high dissolved oxygen. High values of abundance and species richness could be related to the fact that fish species use this habitat as a refuge from the predation during certain periods of their life history.6 In a Neotropical river similar to our system, Echevarría, et al.,10 found that areas with open sand and low habitat structural complexity had higher species richness and trait diversity. They argued that despite habitats with submerged vegetation are more complex that open sand areas, they tend to last less preventing fish communities to reach higher species richness.
We are aware that there could be certain limitations due to sampling bias related to the gears used, especially in littoral areas of the creeks and oxbow lakes. However, we performed a standardized sampling, that yielded a high number of small species which are representative to evaluate the species-habitat associations. De Bie et al.,67 compare aquatic communities of different taxa and found that small freshwater organisms represent strongly habitat-associations due to their dispersal limitation, short generation timer and rapid population growth.
Species composition in the assessed habitats agrees with different studies performed in the Orinoco River Basin, where the orders Characiformes and Cichliformes are the most diverse at family level.68–73 The Bita River basin was characterized for its high species richness, the collected species represented 20% (191 spp.) of the total reported for the Orinoco River Basin (958 spp.) and 50% for the Meta River (380 spp.).75,75 These results position this basin as one of richest in the Neotropical region,69 because its diversity exceeds river basins of equal or greater size, such as Vichada (52 spp.), Arauca (191 spp.) and Cinaruco (155 spp).68,69 These results together with the striking diversity of flora and fauna found by Trujillo and Lasso, 2017 helped to declared in 2018 the Bita River as the first protected river in Colombia under the Ramsar convention.
We thank to the Oficina de Investigaciones y Desarrollo Científico of the Universidad del Tolima, Institute of Biological Research Alexander von Humboldt (IAvH), OMACHA Foundation and WWF Colombia for providing funds for this research through the project "Integrated Management of Water Resources in the Orinoco River Basin". We thank to Saulo Usma-Oviedo (WWF Colombia), Fernando Trujillo (OMACHA Foundation), Carlos Donascimiento and Carlos Lasso (IAvH), Giovani Guevara. and Donald C. Taphorn B. for logistics and taxonomic support.
The author declares there are no conflicts of interest.

©2019 Ospina, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.