eISSN: 2575-906X


Numerous debates exist as to the precise definition of the term "heavy metal" and which elements appropriately fit into such. Several authors rationalized the definition on atomic weight; others, based on specific gravity of greater than 4.0, or more than 5.0 while a few based it on chemical behaviour. Regardless of one’s choice of classification, heavy metal toxicity is a rare diagnosis. However, if undetected or inefficiently managed, heavy metal exposure can lead to remarkably disability and death. This paper gives a succinct and systematic review on the emission, absorption, metabolism and excretion of selected heavy metals. It also delves into their biotoxic effects on the human wellbeing and the ecosystem in general with the mechanisms of their actions. It concludes with the various therapeutic options and management plans for different heavy metal poisoning. This review posits that though heavy metal poisoning could be clinically diagnosed and medically treated, the most appropriate measure is to avoid heavy metal contamination and its subsequent human exposure and toxicity. Thus exposure monitoring and possible intervention for minimizing marginal exposure of humans to heavy metals in the surrounding may be a giant stride towards prevention. National and international synergy is pivot for designing right measures for the prevention of heavy metal toxicity.
Keywords: heavy metals, biotoxicity, bioaccumulation, chemobiokinetics, heavy metals treatment
Exposure to heavy metals has been an issue of public health interest for many decades. Extensive studies on these metals and their toxic impacts have been conducted and periodically revised by international agencies. The utilization of heavy metals by humans has been in existence for several millennia. Although numerous deleterious effects associated with it has been in erudition for several ages, yet heavy metals exposure still rage unabated in some part of the globe, more especially in poor economy nations, albeit emission has dwindled drastically in the last century in the advanced nations of the globe.1 Therefore, the issue of heavy metals contamination is of public health concerns and its crucial anthropogenic emission sources include mining, foundry, chemicals, metallurgy, mechanics, waste incinerators, petrochemicals, fossil fuels combustion and fossil fuels combustion.2
Heavy metals are innately distributed in discrepant concentrations in the ecosystem. They either occurred in elemental form or are associated with other elements in chemical compounds. Heavy metals that are volatile or occurred as fine particulate matter are usually transported over a long range of distance. Each heavy metals toxicity and impacts on food web depend solely on its chemical behaviours and properties. The biogeochemical cycles and innate equilibrium of some heavy metals in the ecosystem have been greatly affected by anthropogenic activities. According to several reports, production of heavy metals viz lead, zinc and copper increased by 10-fold magnitude between 1850 and 1990.3,4 Lead (Pb), Mercury (Hg) and Cadmium (Cd) are of uttermost interest due to their vehement toxicity and deleterious effects on human health. Copious data for these metals in Europe exist in literatures than for others.
It is momentous to stress that heavy metals becomes inimical when its bioaccumulation in living things exceed certain threshold concentration (i.e the dose that makes the effect”).5 Some elements [Zinc (Zn), Copper (Cu), Iron (Fe), Cobalt (Co), Manganese (Mn), Nickel (Ni) and Molybdenum (Mo)] are referred to as micronutrients or trace elements because they are of biochemical importance to animal and plant physiology. Hence they are termed “heavy metal” only when their bio-concentration exceeds that of the threshold concentration and exhibit toxic effects.6,7 The major port of entry of heavy metals to biological system is via inhalation and ingestion. The primary portal of entry in human population is via ingestion.8 Dermal route is a potent means of exposure in humans when the body comes in contact with heavy metals in agriculture, mining, industry, pharmaceuticals, manufacturing or domestic settings. A popular means of exposure in adult is via industrial exposure while in children is via ingestion.9 Juveniles take in poisonous dose via manual-to-oral route through polluted soil or via ingesting items.10 Improper therapy, cracked thermometers and radiological procedure are less popular ways of exposure.11
Heavy metals contamination of the general environment takes place through several routes. Due to their persistence, they tend to infiltrate the environmental compartments, even after many decades of their initial deposition; and pollution of the ecosystem usually occurs through weathering fraction.12 Their bioaccumulation in soil and crops from anthropogenic and natural means and aftermath effects posed vital problems in the food chain through biomagnification. Food safety concerns and possible inimical health risks make this a pivotal environmental concern.13 Common consequences of heavy metals bioaccumulation at biochemical levels include substitution of vital ions, plasma membrane damage, reactions with –SH groups, reactions with phosphate ions and competition with essential metabolites for binding sites.14 In this review paper, attempt is made to give a holistic view of the occurrence, emission, chemobiokinetics and biotoxicity of some selected heavy metals with the view to ascertain their current status, trend and possible protection and remedial practice. It is hoped that this article will spur the interest of researchers, public health professionals, policy makers and the general populace since heavy metals bio-contamination is of public health interest.
What are heavy metals?
There is no universal or conventional criterion for heavy metals definition. Based on the context or premise, diverse meanings may be attributed to the term. In physics, the remarkable criterion for defining heavy metals is atomic number,15 while in metallurgy, it is based on density,16 whereas a chemist is more interested with its chemical behaviour.17 In 2002, it was generally agreed as vague term owning to the adoption of numerous meaning in the last six decades.18
The term “heavy metals” means elements with a relatively high density which is toxic even at minute dose.19 “Heavy metals” is a generic terminology which is applicable to a set of metals and metalloids with atomic density greater than 4 g/cm3, or 5 times or more, denser than water.20,17,1 Nevertheless, heavy metal is intrinsically more of chemical features than specific gravity. Heavy metals include arsenic (As), silver (Ag), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), mercury (Hg), lead (Pb), zinc (Zn), and the platinum group elements.21 When at minimum concentration, these metals are quintessential to the sustainability of numerous physiological and biochemical roles in living organism; however when they exceed certain threshold dosages they become deleterious. Albeit it is widely accepted that heavy metals causes numerous chronic health impacts, yet its exposure persist and is cascading in diverse parts of the globe. They are consequential contaminants in the surrounding and their toxicity pose a nagging problem for the total environment.14,22
Emission of heavy metals
Elemental and compound forms of heavy metals are from both man-made and natural processes. Emission sources which are anthropogenic include several industrial point sources viz past and current mining sites, automobile exhaust fumes.23 Generally, heavy metals are emitted into the ambience during their mining and refining activities.19 Current and old mining field are pivotal source of environmental contamination by heavy metals; and pollution declines proportionally with further distance from mining areas.24 These metals percolate or are usually wash off by acid rain downstream into the oceans. Pollution of water bodies occur largely from mining activities.25,26 These metals are conveyed by water bodies as either hydrated dissolved species or as inherent constituent of suspended sediments, (hydrated species have the highest propensity to cause the significant virulent impacts). They are either deposited in river bed sediments or percolate underground water sources thereby polluting the aquifers most especially well; and the degree of pollution depend largely on closeness of well to mining field. Wells sited near mining fields have been confirmed to have heavy metals concentration exceeding the maximum contamination limit (MCL) specified for drinking water by World Health Organisation.24,27 The permissible limits of selected heavy metals are depicted in Table 1.
|
Heavy metal |
Max conc. in air (mg/m3) |
Max. conc. in sludge (soil) (mg/Kg or ppm) |
Max. conc. In drinking water (mg/l) |
Max conc. in H20 Supporting aquatic life (mg/l or ppm) |
|
Cd |
0.1 – 0.2 |
85 |
0.005 |
0.008δ |
|
Pb |
- - |
420 |
0.01п (0.0) |
0.0058δ |
|
Zn2 |
1, 5* |
7500 |
5 |
0.0766δ |
|
Hg |
- - |
< 1 |
0.002 |
0.05 |
|
Ca |
5 |
Tolerable |
50 |
Tolerable >50 |
|
Ag |
0.01 |
- - |
0 |
0.1 |
|
As |
- - |
- - |
0.01 |
- - |
Table 1 United State Environmental Protection Agency (USEPA) maximum contamination levels for heavy metal concentration in air, soil and water
(Value in bracket is the desirable limit; пWHO; 1adapted from U.S. – OSHA; 2EPA, July 1992; δUSEPA, 1987; Georgia Code, 1993; Florida Code, 1993; Washington Code, 1992; Texas Code, 1991; North Carolina, 1991; *1 for chloride fume, 5 for oxide) fume; - - no guideline available).
The United Nations Economic Commission for Europe (UNECE) 1998 Aarhus Protocol on Heavy Metals was ratified by ending of 2003. The three primary targeted heavy metals in the protocol are Cd, Pb and Hg. In consonance with the protocol, signatories will lessen their emission for these three prioritized elements in relation to their 1990 rates. The Protocol further target to dwindle emissions from selected combustion processes, industrial sources and waste incineration. It is pertinent that a reliable emission profile of heavy metals be made available in order to assess further steps to decrease their environmental exposure; also to elucidate and surmise the source-receptor relationships of heavy metals regionally.
Chemobiokinetics and toxicity effects of selected heavy metals poisoning
Arsenic
Arsenic discovery is acknowledged to the German Scientist Albertus Magnus in 1250. The etymology ‘arsekon” signifies yellow orpiment.28 Arsenic is a metalloid with the chemical symbol As, atomic number 33 and atomic weight 74.92. On the periodic table, Arsenic is the thirty-third (33rd) element. It occurs in nature in metallic state as three allotropes and also in numerous ionic states. Environmental arsenic occurs primarily as sulphide complexes e.g. realgar (As2S2), orpiment (As2S3) and iron pyrites.29 It’s acknowledges as toxic and carcinogenic human agent (ATSDR, 2007). Arsenic top as the highest priority contaminant on the ATSDR/Environmental Protection Agency (EPA) priority list of toxic materials at superfund sites.30 In hydrated state, it occur mainly as arsenate (+5), but more likely as arsenite (+3) in anaerobic conditions. Its usual concentration in natural waters is less than 1 to 2 mg/l; however, the concentration can be significantly elevated in ground waters.
Metabolism of arsenic
Following ingestion of arsenic, it becomes soluble and readily absorbed from the gastrointestinal tract. Arsenate, As (V) as a higher absorption than As (III) arsenite, because arsenate is more permeable through the gastrointestinal tract’s membranes. After absorption, arsenic is conveyed via blood to various body organs, primarily as Monomethylarsonic acid (MMA). The arsenic blood concentration of people with insignificant exposure is within 1 -5g/l As; whereas in soft tissue, it range from 0.01g As/gm. The maximum concentrations is present in nails and hair (0.1 - 1g As/g) where it bioaccumulates.31
There are two processes involved in arsenic metabolism in humans. After cell entry, arsenate is reduced to arsenite. Arsenite afterward undergo methylation give rise to Monomethylarsonic acid (MMA) and Dimethylarsinic acid (DMA); this process takes place mainly in the liver.32 Absorbed arsenic passes to blood stream and undergoes first-pass effect, and afterward distributed to organs/tissues. Once absorption occurs, arsenic combines rapidly with the globin fraction of heamoglobin and thus localises in the blood within 24 hours. Redistribution of arsenic takes place in the spleen, renal, hepatic, pulmonary and gastrointestinal tract, with minimal accumulation in nerve and muscle.
After accumulation of minute arsenic dose, the methylated arsenic (monomethylarsonic acid and dimethylarsinic acid) are excreted alongside organic arsenic residue in the urine. Biologically, the trivalent arsenite is essentially active compare to pentavalent arsenate, including the capability to influence gene magnification in mammalian cells. The biologic fates of arsenate and arsenite differ remarkably. Arsenate invades the cell through the phosphate carrier system and binds to poly-phosphates like adenosine di-phosphate, afterward, undergoes rapid hydrolysis, while arsenite interacts with thiols e.g. glutathione (GSH) and thiol containing proteins. Arsenic is phase out relatively rapidly and mainly through kidney. Urine is the main elimination route for both pentavalent and trivalent inorganic arsenicals.29 Primary routes of exposure and diverse process in arsenic metabolism are illustrated in Figure 1.
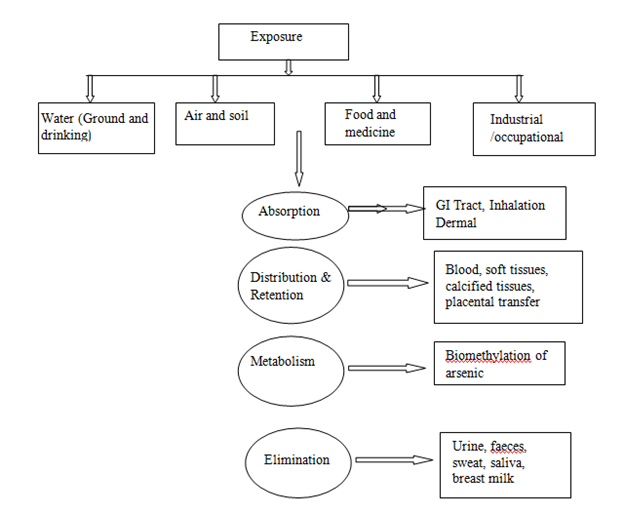
Figure 1 Major routes of exposure and different steps in arsenic metabolism (Source: Chouhan and Flora).29
Health effects of arsenic
Clinical symptoms
The clinical symptoms associated with early period of human arsenic poisoning are usually undefined and proper diagnosis rely primary on recognition of the ailment. Early symptoms observed among people drinking highly arsenic contaminated water, accompanying non-specific symptoms, which are also associated with numerous diverse diseases. These include insomnia, headache, weakness, nightmare, palpitations, fatigue, dizziness, numbness in the extremities, anaemia. Arsenical toxicity (also known as arsenicosis) arises stealthily within a semester or above.
Acute arsenic poisoning usually take place through ingestion of contaminated drink or food and this often requires prompt medical attention. Acute intoxication symptoms manifest within 30 minutes of ingestion but usually prolonged by food. Muscular pains, fatigue, with skin flushing are early clinical symptoms associated with acute arsenic intoxication and to some extent circulatory collapse usually takes place alongside renal impairment and declined urinary output, with nervous maladies. Ultimately, seizures, coma, and death, usually due to shock, may occur. Sub-acute As toxic typically affects the respiratory, gastro-intestinal, cardio-vascular, nervous and haematopoietic system.33,34
Reproductive effects
It has been documented since the past half a century that inorganic arsenic readily access the placenta barrier and inhibits fetal development.35 Chakraborti et al.,36 reported that pregnancy complications were caused by long-time exposure to groundwater As. The authors observed a positive correlation between increased fetal loss & premature delivery and elevated exposure to arsenic. Another study by Hopenhayon et al.,37 observed deleterious effects on the uterine fetus among women exposed to As contaminated drinking water during conception. The impacts of As exposure on pregnancy outcomes via drinking water were also studied by Haiyan and Stuanes.38 They established a viable linkage between long-term As exposure and spontaneous abortion and still birth.
Hematological effects
The hematopoietic system is inimically impacted by both acute and chronic arsenic exposure. Anemia (normochromic normocytic, aplastic and megaloblastic) and leucopenia (granulocytopenia, thrombocytopenia, myeloid, megalodysplasia) are typical effects of poisoning and is reported as a consequence of acute, intermediate and chronic oral exposure to arsenic.33 Mizuta et al.,39 reported anaemia and leukopenia in adults ingesting 3mg As/day in soy sause. Bone marrow depression in human has been linked to relatively high doses of As.40
Cardiovascular effects
Both the heart and peripheral arterial tree usually manifest effects of As toxicity vis cardiovascular aberrations, Reynaud’s disease, myocardial infarction, myocardial depolarization, cardiac arrhythmias, vascular sclerosis. Smith et al.,41 observed induced hypertension among arsenic affected people in Bangladesh. Numerous cases of myocardial infarction and sclerosis among infants who consumed water containing 0.6mg/l arsenic were reported by Zaldivar.42
Carcinogenic effects
Initiation of cancer seen to be the commonest distinct chronic impact of long-time exposure to inorganic As. Epidemiological researches have affirmed a casual association between inorganic arsenic exposure and pulmonary & dermal cancer in man.43
Cadmium
Cadmium was discovered in 1817 simultaneously by German Chemists Fredrick Stromeyer and Herman as an impurity while studying zinc compound.2 Cadmium is mainly found in zinc containing ores but may also be present in lead and copper ores. Cadmium loses its lustre in moist air and rapidly fades by moist ammonia and sulphur dioxide. Cadmium is denoted by the symbol Cd, with atomic number 48 and atomic mass 112.411.
Cadmium produced lethal toxicity even at very low concentration.44 Cadmium and its compounds are relatively soluble in water vis-a-vis other heavy metals. Hence have greater mobility, for instance they are more bio-available and often bioaccumulate in soil.45 Longer duration to cadmium exposure results in diverse types of acute and chronic effects in humans. Cadmium usually tends to bioaccumulate in the kidney of human body causing calcium metabolism disorder, hypercalciuria and renal calculi; while elevated exposure can result in plumonary and prostate cancers.44
Cadmium is emitted to the surrounding via wastewater, fertilizer run-off and atmospheric contamination. Contamination in drinking water may also be attributed to zinc impurities in galvanized pipes.46 The primary source of daily exposure to cadmium is through food approximately 10 to 35mg. An additional important means of cadmium exposure is via cigarette or tobacco smoking.47
Mechanism of cadmium toxicity
The science of cadmium poisoning is still vague but its cytological outcomes are well established.48 The concentration of cadmium magnifies by 3,000 fold on binding to cystein-rich protein such as metallothionein. In the liver, the cystein-metallothionein complex result in hepatotoxicity and afterward migrates to the kidney and bioaccumulates in the renal tissue causing nephrotoxicity. Cadmium has the potential to bind to several amino acids ligands and resulting to iron deficiency.49 Cadmium can substitute zinc present in metallothionein due to similar oxidation number, thus impeding it from performing free radical scavenger role in the cell.
Environmental effects of cadmium
Bioaccumulation of cadmium readily occurred in numerous organisms, especially in microorganisms and molluscs where the bio-concentration index are in the magnitude of thousands. Most organism display mild to moderate cadmium concentration level of less than 100ug/g however soil invertebrates can concentrate cadmium significantly. In animals, cadmium concentration takes place in internal organs. It is markedly greater in the following order: kidney > liver > muscle. Cadmium concentration increase significantly with age. Cadmium is a non-essential plant and animal element.50,51
Health effects of cadmium
Based on present erudition, kidney impairment is likely the critical health consequence, both in the general population and among occupationally exposed workers.44 Cadmium accumulation in the kidney (in the renal cortex) results in kidney derangement with impaired reabsorption of, for instance, amino acids, proteins, and glucose.52 According to Jarup et al.44 osteoporosis has been noticed as the profound outcome of cadmium exposure in both human and animal studies but the relevance of such effect still remains nebulous. High occupational exposure may be accompanied by pulmonary variations majorly characterized by chronic obstructive airway ailments. With continuum in the cadmium exposure, early minor variations in ventilatory function tests may aggravate to respiratory incompetence. A rise in death rate from obstructive pulmonary diseases has been reported among workers with history of elevated cadmium exposure.50,51 The International Agency for Research on Cancer (IARC) categories cadmium in Class 1.53 Pulmonary and prostate cancer has been linked to occupational exposure to cadmium. Based on current survey, epidemiological data has established a stronger positive correlation between cadmium and pulmonary cancer than with prostate cancer.54
Chromium
Chromium was discovered in France by a scientist called Louis Vauquelin in 1797. The name is coined from the Greek word “Chroma” implying colour due to the numerous colours produced by chromium compounds. Chromium is a metallic element designated by symbol Cr, atomic number 24, atomic weight 51.9961.55 Chromium has variable oxidation states from chromium (-II) to chromium (+VI). Chromium compounds are stable in the trivalent state, with the hexavalent form being the second most stable state. The chromium (III) compounds are sparingly soluble in water while the chromium (VI) compounds are readily soluble in water. Chromium (Cr) occurred naturally in ores, soils and plants.56
Mechanism of chromium toxicity
Trivalent chromium Cr(III) is usually innocuous in the environment owning to its deficient membrane porosity. Contrarily, hexavalent chromium Cr(VI), is more potent in cell membrane penetration by providing entrance for isoelectric and isostructural anions vis SO42– and HPO4 2– channels and are engulfed by phagocytosis. Glutathione is responsible for the stabilization of the pentavalent form and thus if intracellular reduction of Cr(VI) takes place close to the target site, it usually cause Cr activation or otherwise vice-versa. The reactions between Cr(VI) and biological reductants like thiols and ascorbate leads to reactive oxygen species production, finally resulting in cell oxidative stress causing DNA and proteins impairment (Stohs and Bagchi, 1995). Based on previous studies, Cr(VI) is reported as a human carcinogen and highly deleterious than Cr(III), based on Cr(VI) invasion of cells more rapidly compare to Cr(III) and is ultimately reduced to Cr(III).57,58
Effects of chromium on the environment
Acute exposure to chromium can lead to skin irritation and ulceration; while renal and hepatic damages, as well as impairment of the nervous and circulatory system can arise from chronic exposure. Accumulation of chromium usually takes place in aquatic organisms, thus contributing to the risks of consuming fish previously exposed to elevated concentrations of chromium.59
Chromium effects on plants and animals
Effects on plants
Hexavalent Cr can negatively influence plant physiological processes such as photosynthesis and resultantly imped plant growth and development.59
Effects on animals
Chromium can negatively affect respiratory and reproductive activities in animals, interferes with hormone production, and even lead to death.58 Chromium can accumulate in organism tissues; albeit it is established to accumulate via food chain.57
Health risks of chromium
Chromium occurs in binary forms in water: trivalent chromium (chromium III) and hexavalent chromium (chromium VI). Chromium III and chromium VI exerts differs toxicity effects. Chromium III is frequently present in water; it is vital to human nutrition and is considered non-poisonous. When chlorine combines with chromium III, it turns to chromium VI.56 Exposure to chromium VI in drinking water at levels above 0.05 mg/L in may result in nausea, abdominal pain, indigestion, convulsions, and liver and renal impairment. Human health is only at risk through ingestion via teeth brushing, drinking, and cooking. Well water containing chromium at level exceeding 0.05 mg/L may safely be utilised for bathing, washing of hands and dish.58
Reproductive effects
There is paucity of data to support that Chromium (VI) compounds has deleterious impact on male reproductive system. In a study of 21 electroplating workers in Henan, China exposed to chromium (VI), a remarkable decrease in sperm count and motility and essentially rise in follicle stimulatory hormone levels was observed.60
Carcinogenicity
The international agency for research on cancer (IARC) have categorised chromium (VI) as human carcinogen based on numerous established evidences as observed among workers exposed to chromate.61 Epidemiology studies firmly established the relationship between pulmonary cancers and chromium (VI) compound exposure (COC, 2004). This report is corroborated by findings from studies conducted among workers in chromium production industries which consistently expressed lung cancers.
Lead
Lead is a bluish-white lustrous metal, which is very soft, highly malleable, ductile, and a relatively poor conductor of electricity. It shows high resistance to corrosion but tarnishes upon exposure to air.62 It carries the chemical symbol Pb, with atomic number 82 and mass number 207.2.
Exposure routes
Paint
Some lead compounds are widely utilised in paints, which is is a primary source of lead exposure in children.63 Deteriorating lead paints can generates toxic lead levels in household dust and soil.64
Soil
Lead contamination in soil may arise from degraded lead paint, residues from lead-containing gasoline, spelt engine oil or pesticides used in previous, contaminated landfills or from adjacent industries such as foundries or smelters.65
Water
Lead from atmosphere or soil can percolate in groundwater.66 Lead contaminates drinking water through corrosion of lead solder that connects the pipes or brass faucets. In US, drinking water account for about 14-20% of total lead exposure.67
Occupational exposure
In adults, occupational exposure is the primary causative of lead poisoning.68 Majority of the people affected are those working in facilities that manufacture diverse lead containing products.69 Lead miners, smelters, plumbers, auto mechanics, glass manufacturers, construction workers, battery manufacturers and recyclers and plastic manufacturers are at risk for lead exposure.70
Mechanisms of lead toxicity
Toxicity of lead metal in biological system is sequel by ionic mechanism and oxidative stress. Several workers have demonstrated that oxidation in biological systems is initiated by disequilibrium in the free radicals generation and antioxidant production necessary to cause reactive intermediates detoxification and resulting damage repair. Antioxidant, for instance glutathione, protects the cell from free radicals like H2O2. Interference of lead causes the level of reactive oxygen species (ROS) to rise while the level of antioxidants declines. Since glutathione occurs both as reduced (GSH) and oxidized (GSSG) state, the reduced state of glutathione gives its reducing equivalents (H+ + e−) from its thiol groups of cystein to ROS in order to cause stability. Under the influence of the enzyme glutathione peroxidase, reduced glutathione readily binds with another molecule of glutathione after electron donation and give rise to glutathione disulfide (GSSG). About 90% of the total glutathione content is in the reduced form (GSH) while the remaining 10% accounted for by the oxidized form (GSSG) under ideal state. Rather in the oxidative stress state, the GSSG concentration surpasses the GSH concentration. Another biomarker for oxidative stress is lipid peroxidation, by which free radical takes electron from lipid molecules inherent in the cell membrane, which resultantly give rise to lipid peroxidation.71,72 At elevated levels, ROS may result in structural deformation of cells, proteins, nucleic acid, membranes and lipids, culminating in a stressed state for the cells.73
The ionic mechanism of lead toxicity is caused primarily by the capability of lead metal ions to substitute other bivalent cations vis Ca2+, Mg2+, Fe2+ and monovalent cations vis Na+, which consequentially causes disturbance in cell biological metabolism. The ionic mechanism of lead toxicity results in momentous variation in several biological activities. Lead can substitute calcium even in picomolar concentration affecting protein kinase C, which controls excitation of neurons and memory storage.74,75
Effects of lead in the environment
Human vulnerability to lead can lead to numerous biological impacts depending largely on the concentration and exposure timing. Different effects arise from a wide diverse of concentrations, with the developing foetus and infant being more vulnerable than the adult.71 Elevated levels of exposure may lead to deleterious biochemical effects in humans which may then cause impairment in haemoglobin synthesis, affect the alimentary, and short and long-term damages to the nerves. Lead poisoning, which must be very severe before it results in visible ailments, is now very rare indeed. There is convincing proof that exposure to intermediate concentrations of lead can result in minute, nebulous, subclinical effects, most especially on children neuropsychological development.59 Nevertheless, majority of people are exposed to huge proportion of lead via ingestion of food, while other consequential means may be through drinking water in lead pipe and plumbosolvent water, air from nearby emission source, aerosols from stale monuments or polluted land. Atmospheric lead adds significant to lead concentrations in food via dust deposition and precipitation containing the metal falling on soil and crops.
Health effects of lead
Lead is highly toxic metal affecting virtually all organs and biological system, particularly the nervous system and likewise the reproductive and other organ systems.76
Renal effects
Kidney impairment takes place following high level lead exposure, and scientific fact surmise that kidney damage can occur even at minimal concentration. The deleterious impacts of lead results in nephropathy and is indicted in Fanconi syndrome – derangement of proximal convoluted tubules.77
Cardiovascular effects
Evidences are replete that exposure to lead is correlated to elevated blood pressure and evidences have also linked lead exposure to coronary heart disease, heart beat discrepancy and mortality due to stroke.78
Reproductive effects
Lead affects both the reproductive system of male and female. In man, when blood lead is greater than 40µg/dL, there is reduction in sperm count and variations takes place in sperm volume, morphology and motility.77 High blood lead concentration can result in miscarriage, reduced birth weight, premature and developmental anomalies during childhood.79 Lead crosses the placenta barrier and into breast milk, resulting in equal blood lead level in both mothers and progenies. Mobilisation of lead in mother’s bone following metabolic change arising from conception can result in the poison of the fetus in-utero.
Nervous effects
Lead attacks both peripheral nervous system (especially motor nerves) and central nervous system. Peripheral nervous system damages are highly pronounced among adults while central nervous impacts are greatly evident among juveniles.80 Lead causes degeneration of the axon of nerve cells and makes them unmyelinated especially those of the brain.81 Lead is able to cross the endothelia cells at the blood brain barrier due to its substitution with calcium ion and subsequent uptake by calcium ATPase pump.82 Juveniles are more vulnerable to lead neurotoxicity than adults because lead contamination mingles with the proper maturation of an infant’s brain and nervous coordination.83
Cancer effects
The human epidemiological data on lead carcinogenicity is regarded as unconvincing. The assessment of carcinogenic risk from lead exposure has been based mainly on observations from epidemiological studies, experimental animal studies and acute exposures. The IARC has categorised lead and inorganic lead compounds as potential human carcinogen, citing insufficient evidence for humans carcinogenicity but adequate facts are suffice for carcinogenicity in experimental animals.61
Effects on children
A developing uterine fetus in a pregnant mother with high blood lead is likewise vulnerable to lead poisoning, and has propensity of premature birth or greater risk of reduced fetal weight. Children are more sensitive to lead poisoning due to their stature that still experiencing continuum maturation.84 Absorption of lead takes place rapidly than in adults, resulting in more harmful consequences. Infants, particularly in their developmental stage are frequently in contact with the floor and hence more susceptible to ingestion and inhalation of dust contaminated lead.65 The distinct manifestations in children are appetite loss, abdominal pain, nausea, weight reduction, constipation, anaemia, kidney impairment, lethargy, cognitive impairments and behavioral derangements.84 Tardy development of proper childhood behaviours like talking and words usage and permanent mental retardation are frequently manifested.85
Aluminium
Aluminium is the third plethora element and is found naturally in the soil, hydrosphere and lithosphere.86 Aluminium extraction and processing increased the anthropogenic level in biosphere.87 Current studies in environmental toxicology showed that aluminium may pose a significant threat to biotic organisms as aetiologic agent.88 Aluminium toxicity is grossly affected by several parameters, such as water and organic matter content. Aluminium toxicity rises with reduction in pH. Toxic aluminium ions mobilization arises from variation in soil and water pH caused by acid rains and precipitated by elevated acidification of the ambient air, which resultantly has an inimical impact on the ecosystem. This is evident in forests drying, plant poisoning, crop reduction, mortality of aquatic animals, and also by numerous aberrations in biological function.88 Owning to aluminium toxicity in top soil, the crop production was restrained by 67% across the globe. Acidity of the soils causes erosion of silicon leaving remnant of solidified aluminium termed aluminium oxyhydroxides, which release phytotoxic Al3+ popularly referred as Al (OH)63+ in soil.89 The interaction of Al3+ with apoplastic, plasma membrane, and symplastic targets results in toxicity and distortion of the structural and functional activities of the plants. The usual symptoms are chlorosis, pulping, root growth impedance, necrosis, cytogenic and foliar aberration.86
Elevated concentration of aluminium is highly lethal to aquatic organisms, particularly for gill breathing organisms (e.g fish), causing osmoregulatory distortion and hemolymph ions failure. Monomeric form of aluminium in fish impedes the activity of gill enzyme, which is vital for ions uptake.90 Also affected by Al toxicity are aquatic organisms vis seaweeds and crawfish.91 Aluminium is poisonous and devoid of any bio-importance to microbes92 and nerve, bone and haemopoietic cells.88 Aluminium impedes enzymes such as hexokinase, phosphodiesterase, alkali phosphatase and phosphoxidase because of its stronger affinity to nucleic acids.
Mechanism of aluminium toxicity
Aluminium disrupts most structural and cellular activities.93 The precise process by which aluminium is absorbed via gastrointestinal tract is still nebulous. Referencing existing literatures, it is arduous to allot an exact duration for aluminium toxicity since the duration of the diagnosis of its manifestations varies remarkably.94 In humans Mg2+ and Fe3+ are surrogated by Al3+, which leads to several imbalances linked with intercellular communication, cellular growth and secretory functions.93 The neurological deviations triggered by aluminium can be likening to the degenerative lesions seen among Alzheimer patients. The grievous difficulties associated with aluminium poisoning are neurological maladies vis neuronal atrophy in the locus ceruleus, substantia nigra and striatum.88,92
Iron
Iron is one of the most important elements required by all living organisms for growth and survival.95,96 It is an essential constituent of organisms like algae and of enzymes such as cytochromes and catalase, and likewise protein-binding oxygen, such as hemoglobin and myoglobin.97 Sulphuric acid production and ferrous (Fe2+) discharge occurs by oxidation of iron pyrites (FeS2) that are typical of coal seams.96 The simplified oxidation reactions for ferrous and ferric iron are depicted below:
2FeS2 + 7O2 → 2FeSO4 + H2SO4 (ferrous)
4FeSO4 + O2 + 10H2O → 4Fe(OH)3 + 4H2SO4(ferric)
The level of dissolved iron in the deep ocean is usually 0.6nM or 33.5 × 10−9 mg/L; in freshwater the estimated concentration is 5μg/L–ICP, while in groundwater is about 20 mg/L.95 The biomass of periphyton, benthic invertebrates and fish diversity are largely influenced by the explicit and implicit impacts of iron contamination.97 Iron precipitation will result in remarkable impairment by clogging and impeding fishes’ respiration.95 Lowland rice production was significantly influenced by elevated iron (Fe2+) concentrations in irrigated soil.98 The properties associated with rice toxicity include elevated Fe2+ uptake by roots, acropetal translocation into leaves and declined yield.99
Mechanism of iron toxicity
Numerous ranges of inimical unbound radicals are produced when the absorbed iron fails to bind to the protein, consequently having severe impacts on iron concentration in mammalian cells and physiological fluids. The free iron results to lipid peroxidation; which in turns lead to deleterious impairment of cellular organelles.100 Excess intake of iron produce diverse ranges of free radicals that are acknowledged to be potential cellular damage. The iron generates hydrogen free radicals that attack DNA, leading to cellular destruction, genetic aberration and malignancy which in turn trigger myriads of anomalies in the heart, liver and brain.101
Overview of treatment regimens
Therapies to eliminate heavy metals poisoning from the humans’ system include chelation and decontamination techniques, and supportive measures, usually apply in synergy. The therapies can be highly complicated and individualized, restricted to individual’s specific needs and demanding the dexterity of a pundit, often a conglomerate of expatriates.
Chelating agents’ administration is the routine used in treatment of heavy metals poisoning in humans.102 These chemical agents include as CaNa2 EDTA (calcium disodium ethylenediaminetetraacetate) that transform heavy metals to chemically inert states which can afterward be excreted from the body without further interference. Chelates have side effects and can obliterate essential elements from the body. Base on this reason, vitamin and mineral supplements are often co-administered.103
Chelation therapy
Chelation is a chemical technique with diverse applications in medicine, environment, chemistry and so forth. Chelation therapy, simply termed as the mechanism by which a molecule surrounds and adheres to a metal and eliminates it from tissue (Dr. Joseph F. Smith Medical Library 2001). Based on the nature of drug administered, chelating agents are given orally, intramuscularly, or intravenously; and are excreted from the body through the kidney via urine.10 The decision to chelate is solely the responsibility of the pundit in chelation therapy.
Chelating agents
A popular compound employed in chelation therapy is dimercaprol (also known as BAL or British Anti-Lewisite). Oral chelating agents used as substitute to BAL are 2,3-demercaptosuccinic acid (DMSA), dimercaptopropane sulfonate (DMPS), and D-penicillamine (ATSDR MMG). Another agent, deferoxamine, is frequently used to chelate iron. Ethylenediamintetraacetic acid (ETDA) was among the premiere chelators developed and has strong affinity for lead.
BAL (British Anti-Lewisite): is a chelating agent administered via injection in the treatment of acute poisoning by certain heavy metals (e.g., arsenic, lead, mercury, gold, bismuth, and antimony). BAL side effects include gastrointestinal disorders; headaches; muscle spasms, bronchoconstriction; and profuse sweating.104 It is considered one of the most lethal chelating agents.31
DMSA (Dimercaptosuccinic Acid): DMSA is analogical to BAL and use for treating toxicity involving lead and mercury. Contraindications to the usage of DMSA are preexisting renal or hepatic ailment and conception. It is paramount to drink a lot of water. DMSA cannot be administered alongside with ETDA or Dpenicillamine.105
DMPS (Dimercaptopropane sulfonate): DMPS is partial similar to BAL. It functional and manifest numerous side effects compare to DMSA (Ford, 2006). D-penicillamine is used for the treatment of arsenic and mercury toxicity. Prohibitions include penicillin, digoxin, kidney incompetence, and conception.31
Deferoxamine: Deferoxamine is used as an iron and aluminium chelator, particularly in acute iron poisoning among infants. Deferoxamine is given via injection or intravenously. Contraindications include conception or breast feeding, and renal ailment.106
EDTA (Ethylenediamintetraacetic Acid, Edetate Disodium): EDTA has an affinity for lead. It is commonly administered as a second-line of treatment in conjunction with BAL and given by intravenous infusion. Severe effects may involve seizures, distorted heartbeat, skin rashes, pyrexia, and hematuria.107 EDTA is prohibited during conception and in the case of renal ailment.108
A succinct summary of the various chelating agent, toxin, routes of administration, and drugs for the treatment regimens of several heavy metals toxicity is shown in Table 2.
|
Chelating agent |
Toxin |
Route** |
Drug |
|
Dimercaprol (BAL) |
Arsenic |
i.m. |
Dimercaptol |
|
Lead |
Injection B.P. |
||
|
Mercury (inorganic)* |
BAL in Oil |
||
|
Dimercaptosiccinic acid (DMSA) |
Arsenic |
p.o. |
Chemet |
|
(Succimer) |
Lead |
||
|
Mercury |
|||
|
Dimercaptopropanesulfonate(DMPS) |
Arsenic |
p.o. |
Bulk form |
|
i.m. |
(for compounding by pharmacists) |
||
|
D-pencillamine |
Arsenic |
p.o. |
Metalcaptase |
|
Mercury |
Pencillamine |
||
|
Lead |
Cuprimine |
||
|
Depen |
|||
|
Ethylenediamintetra- acetic acid (EDTA) |
Lead |
IV |
Chealamide |
|
(Edetate disodium) |
|
Versenate |
|
Table 2 The following table encapsulates the chelating agents, the type of heavy metals toxicity, their route of administration, and their brand name
*Not methylmercury poisoning.
**Under supervision of a physician: i.m., intramuscular; p.o., peroral or by mouth; IV, intravenous.
Source: Data from Ferner;107Kosnett;109 Howland;104-106,108 Mowry.31
Table 3 below summarizes the typical presentations of frequently encountered metals toxicity and their treatment.110–112
|
Metal |
Acute |
Chronic |
Toxic cconcentration |
Treatment |
|
Arsenic |
Nausea, vomiting, "rice-water" diarrhea, encephalopath, MODS, LoQTS, painful neuropathy |
Diabetes, hypopigmentation/ hyperkeratosis, cancer: lung, bladder, skin, encephalopathy |
24-h urine: 0 ≥50 µg/L urine, or |
BAL (acute, symptomatic) Succimer 100 µg/g creatinine DMPS (Europe) |
|
Bismuth |
Renal failure; acute tubular necrosis |
Diffuse myoclonic encephalopathy |
No clear reference standard |
* |
|
Cadmium |
Pneumonitis (oxide fumes) |
Proteinuria, lung cancer, osteomalacia |
Proteinuria and/or ≥15 µg/ g creatinine |
* |
|
Chromium |
GI hemorrhage, hemolysis, acute renal failure (Cr6+ ingestion) |
Pulmonary fibrosis, lung cancer (inhalation) |
No clear reference standard |
NAC (experimental) |
|
Cobalt |
Beer drinker’s (dilated) cardiomyopathy |
Pneumoconiosis (inhaled); goiter |
Normal excretion: 0.1-1.2 µg/L (serum) 0.1-2.2 µg/L (urine) |
NAC CaNa2 EDTA |
|
Copper |
Blue vomitus, GI irritation/ hemorrhage, hemolysis, MODS (ingested); MFF (inhaled) |
vineyard sprayer’s lung (inhaled); Wilson disease (hepatic and basal ganglia degeneration) |
Normal excretion: 25 µg/24 h (urine) |
BAL D-Penicillamine Succimer |
|
Iron |
Vomiting, GI hemorrhage, cardiac depression, metabolic acidosis |
Hepatic cirrhosis |
Nontoxic: < 300 µg/dL Severe: >500 µg/dL |
Deferoxamine |
|
Lead |
Nausea, vomiting, encephalopathy (headache, seizures, ataxia, obtundation) |
Encephalopathy, anemia, abdominal pain, nephropathy, foot-drop/ wrist-drop |
Pediatric: symptoms or [Pb] ≥45 µ/dL (blood); Adult: symptoms or [Pb] ≥70 µ/dL |
BAL CaNa2 EDTA Succimer |
|
Manganese |
MFF (inhaled) |
Parkinson-like syndrome, respiratory, neuropsychiatric |
No clear reference standard |
* |
|
Mercury |
Elemental (inhaled): fever, vomiting, diarrhea, ALI; |
Nausea, metallic taste, gingivo-stomatitis, tremor, neurasthenia, nephrotic syndrome; hypersensitivity (Pink disease) |
Background exposure "normal" limits: |
BAL |
|
Inorganic salts (ingestion): caustic gastroenteritis |
10 µg/L (whole blood); 20 µg/L (24-h urine) |
Succimer DMPS (Europe) |
||
|
Nickel |
Dermatitis; nickel carbonyl: myocarditis, ALI, encephalopathy |
Occupational (inhaled): pulmonary fibrosis, reduced sperm count, nasopharyngeal tumors |
Excessive exposure: ≥8 µg/L (blood) Severe poisoning: ≥500 µg/L (8-h urine) |
* |
|
Thallium |
Early: Vomiting, diarrhea, painful neuropathy, coma, autonomic instability, MODS |
Late findings: Alopecia, Mees lines, residual neurologic symptoms |
Toxic: >3 µg/L (blood) |
MDAC Prussian blue |
|
Zinc |
MFF (oxide fumes); vomiting, diarrhea, abdominal pain (ingestion) |
Copper deficiency: anemia, neurologic degeneration, osteoporosis |
Normal range: 0.6-1.1 mg/L (plasma) 10-14 mg/L (red cells) |
* |
Table 3 Typical Presentation of frequently encountered metals toxicity and their treatment
*No accepted chelation regimen; contact a medical toxicologist regarding treatment plan.
MODS, multi-organ dysfunction syndrome; LoQTS, long QT syndrome; ALI, acute lung injury; ATN, acute tubular necrosis; ARF, acute renal failure; DMPS, 2,3-dimercapto-1-propane-sulfonic acid; CaNa2 EDTA, edetate calcium disodium; MDAC, multi-dose activated charcoal; NAC, N -acetylcysteine.
In this article, we reviewed the emission, chemobiokinetics, toxicity effect as well as the various therapeutic measures for various heavy metals poisoning. Heavy metals are in diverse forms indispensable to human particularly in industrial settings. They are released into the surrounding through man-made and innate phenomenon. But the biotoxicity associated with the indiscriminate exposure to heavy metals may be deleterious to human health and distort normalcy, if not even life terminal, thus could not be undermined. They often behave as a pseudo substance in the biological system while at certain instance impedes metabolic activities. Despite the fact that heavy metals poisoning can be clinically diagnosed and medically treated, the most viable choice is to avoid heavy metals contamination and their subsequent exposure and poisoning. This review opines that failure to control the exposure will lead to deleterious consequences in the future due to the inimical impacts inflicted by heavy metals poisoning. This article also posit that exposure monitoring and possible intervention to minimizing marginal exposure of humans to heavy metals in the surrounding can become a giant stride in a bid to achieve prevention. National and international synergy is pivot for the development of suitable measures in the prevention of heavy metal toxicity.
The authors sincerely acknowledge the efforts of all those who had contributed immensely to the fields of environmental toxicology and environmental medicine.
The authors declare no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.