eISSN: 2575-906X


Research Article Volume 2 Issue 4
1Departamento de Producción Animal, Universidad de Buenos Aires (FAUBA), Argentina
2Laboratorio de Anatomía Vegetal, Museo Argentino de Ciencias Naturales Bernadino Rivadavia, Argentina
3Laboratorio de Ecología de Pastizales, Museo Argentino de Ciencias Naturales Bernadino Rivadavia, Argentina
Correspondence: Fernández Pepi, Departamento de Producción Animal, Universidad de Buenos Aires, Facultad de Agronomía (FAUBA), Av San Martín 4453 (C1417DSE), Ciudad Autónoma de Buenos Aires, Argentina
Received: June 13, 2018 | Published: July 11, 2018
Citation: Fernández MG, Arriaga MO, Stampacchio ML, et al. Botanical composition of sheep diet in two contrasting environments at Tierra del Fuego steppe (Argentina). Biodiversity Int J. 2018;2(4):316-320. DOI: 10.15406/bij.2018.02.00080
The botanical composition of the diet of sheep in two contrasting environments of the fueguian steppe was compared by means of a microscopic analysis of feces, during the spring and summer 2006. In both areas of study, grasses showed to be the most important group in the diet of both locations and of both seasons, followed by native dicotyledonous. In addition, it was found that in the abandoned pasture of Cullen ranch, the intake of Hieracium pilosella, an invasive species, is high. Management strategies should be targeted to the conservation of grasses which are the life forms that are most susceptible to over grazing disturbance. Besides, these results contribute to the stage of advancement of the invasive species H. pilosella in the fueguian steppe and to the knowledge of the incorporation of this species in the diet of livestock, and possibly in the diets of native herbivores.
Keywords: herbivory, diet selection, sheep, fueguian steppe
The relationship between plant’s features and different disturbances has received great attention1,2 mainly due to changes in plant species richness and composition associated with overgrazing.3 It has been shown that species with different traits often respond differently to grazing, but the emerging patterns tend to vary among studies.4‒11 Pasturing by cattle frequently brings about changes in the structure and the operation of the natural pastures, affecting key processes such as the primary and secondary productivity, changes in the vegetation, cycle of nutrients.12,13 botanical composition changes are among the most remarkable modifications observed in pastures as a consequence of the replacement of palatable by non- palatable species.4,13‒18 Selective herbivory is a dominant mechanism, as species consumed less frequently or intensively have a competitive advantage as they substitute those species consumed more severely.19‒21 In addition, soil tilling associated with the implantation of pastures and other methods may facilitate the colonization of exotic weeds.22‒24 The presence of weeds usually reduces the amount and quality of available fodder, increases the handling costs and may harm animal responses.25 The introduction of domestic animals in the Patagonia region, primarily ovine cattle occurred at the beginning of the last century when the early settlers handled the natural grasslands applying experiences gained in different ecosystems.26 This management brought about deep changes to the soil and flora composition.27‒29 Invasion of Hieracium pilosella L. (Asteraceae) in the Austral Patagonia30,31 is a successful invasive species32,33,34 associated to overgrazing since it is highly competitive spreading rapidly by means of vegetative growth of stolons and rhizomes and through mono-specific feeding patches formations. Its greatest economic impact is the decrease of primary and secondary productivity caused by the replacement of native and palatable species in the original grasslands.36‒39 In the province of “Tierra del Fuego” (Argentina) the current invaded area is also overcome by fescue grasslands (Festuca gracillima, Hooker f.) and “Mata Negra” shrub (Chiliotrichum diffusum, Forster f.; O. Kunze;).31,40 At present, the invasion of H. pilosela is wide-ranging in the step region, where it commonly replaces native species from the inter-coironal or the inter-spaces between bushes. These locations are occupied naturally by a large number of little native grasses and naturalized species which represent a key contribution to diversity and to the pastoral value of that community. The knowledge of the botanical composition of the herbivorous diets is necessary in order to be able to decide over land handling alternatives. Our purpose was to compare sheep diets in two areas of the Fueguian steppe: a natural grassland (Maria Behety ranch: MB) and an old pasture (Cullen ranch: EC), taking into account the modification of vegetal communities brought about by different managements handlings.
Study area
The study was conducted in two branches located in the Fueguian steppe. Maria Behety ranch (MB) is placed 15km northwest from the city of “Rio Grande” in “Tierra del Fuego” (53°47´S 67°42´W; Figure 1). The climate varies from semi-arid to sub-humid with oceanic features.41 The average annual rainfall is 371mm with a deficiency in the ground available water between November and mid-April. The dominant vegetation are Coiron grasslands (Festuca gracillima), with areas dominated by Chilotrichum difussun and Empetrum rubrum. In the low-lying areas there are meadows containing hydrophytic vegetation.42
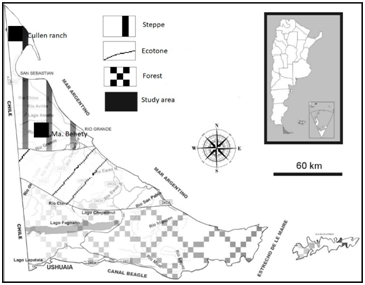
Figure 1 Natural environments of the Tierra del Fuego province: steppe, ecotone and forests. In the steppe is where the study areas are located: MB, MaríaBehety Ranch; EC, Cullen Ranch.
Cullen ranch (EC) is located north from the Fueguian steppe (52°55’ S, 68°33’ W; Figure 1). It has a pastureland (c.a. 1220 ha), containing an area which has been used with pastures for 30 years, actually covered with Hieracium pilosella (above 15%;45).The mean annual rainfall (over the last 100 years) measured close to the ranch’s minor is 335mm, and the month’s average temperature during the coldest month is 0°C and reaches 10°C in the warmest month. Since this area is a wavy relief, it has canyons that limit to low and humid plains ("Vegas") with abundant herbaceous vegetation composed of different species of grasses (Festuca spp., Poa spp., Bromus spp., Agrostis spp.), Forbs (Taraxacum officinale, Caltha sagittata), sedges (Carex spp.) and rushes (Juncus spp. and Luzula spp.). The highlands were covered generally by the plant species "Coirón" (Festuca gracillima), Chiliotrichum difussum and "Murtilla" (Empetrum rubrum).43
Floristic data
To determine the floral and diets compositions, plants of the present communities and sheep faeces were collected during the spring and the summer of 2006 at both locations. The plants which were collected were identified using the reference flora.44‒46 Species were grouped according to their life forms into 8 categories: (sensu 51): cushion (CU), dwarf shrub (DS), tall shrub (TS), grasses (GR), native herb dicotyledons (ND), exotic herb dicotyledons (ED), introduced herb dicotyledons to improvee grasslands (ID), graminoids (GRA).
Sampling and treatment of feces
Food habits of sheep were studied during spring and summer by randomly collecting 1-5 to dung units of every dropping at each feeding area. Faeces samples were spread over paper and dried at ambient temperature for several days and then put into paper bags. Later on, the grounded samples were boiled with 5% NaOH for 1-2 minutes and then rinsed with NaClO, bleached for a few minutes, and thoroughly rinsed in water again. Three slides were made from each sample for microscopic observation, 9 preparations were made per stay, for each station sampled, and 20 optical fields were quantified for each one.
Data Analysis
The floristic composition of a sheep’s diet can be recognized by the relative frequencies of species infaecal samples by means of a microscopic recognition of the epidermis with their cuticle47 and classified by forms of life, location, and season of the year. Plants were identified at species level, but genus or other taxonomic categories were used too when possible. The diversity of plant species in the diet was estimated by the Shannon Diversity Index (H),48 and the species similarity in the diets between seasons and locations by the Sorensen index (SI).49 Diet composition within and among communities and seasons was studied by principal component analysis (expressing every species consumption as proportion of the total of species consumed). Analyses were performed using the program PAST.50
Applied formulas
Where Pi=relative abundance and ln=natural logarithm.
Where C=number of common species between Gi and Si diets; Gi=number of species in the diet of geese, and Si=number of species in sheep diet. The values as (0-0.25) indicate low similarity, (0.26-0.50) moderate similarity, (0.51-0.75) high similarity and (0-76-1) full similarity.
There was a great similarity in botanical dietary composition between periods studied in EC and MB. While comparing the SI taking into account the same period in between sites, the spring period is the most similar in floristic composition (Table 1). The most significant differences were noticed in the frequency of ingestion of some species of grasses, native herbaceous dicotyledons, exotic herbaceous dicotyledons, herbaceous dicotyledons invader and sedges and rushes, being the two first, the most frequently consumed. The diversity and the evenness of the diet tend to be greater during two periods EC, which corresponds to modified pasture (Table 2). The values of these indices show significant differences in MB throughout the summer period compared to MB during spring, and EC in both periods (p•0.005), having MB in summer the least diverse composition (Table 2).
|
MB-SU |
EC-SP |
MB-SP |
0.39 |
0.76 |
EC-SU |
0.52 |
0.83 |
Table 1 Sörensen’s Index values calculated to compare the similarity in the floristic composition of the diets. The values are interpreted following as (0-0.25) indicate low similarity, (0.26-0.50) moderate similarity, (0.51-0.75) high similarity and (0-76-1) full similarity
MB, Ma Behety; EC, Cullen; SU, summer; SP, spring
|
MB-Nov |
MB-Feb |
EC-Nov |
EC-Feb |
H |
3.30±0.19 |
2.70±0.28 |
3.79±0.18 |
3.33±0.31 |
Table 2 Diversity index (H) (Average and error values) of sheep’s diets, during spring and summer, in both studies areas
MB, Ma. Behety; EC, Cullen; SU, summer; SP, spring
In the area corresponding to the old pasture (EC), the life forms consumed in both periods were GR, ND, GRA and ED, being the frequency of consumption of GR the highest in both periods. The other three life forms showed significant differences, though ND showed the highest in spring and the other two in summer (Figure 2). Whereas the species in GR category with significant differences were: Agrostis sp., Elymus sp., Festuca sp., Poa sp., being the three first more frequently preferred by sheep in summer, while Poa sp. was in the spring period. The form life ND with significant differences: Acaena sp., which is more common in the summer intake, as Valeriana sedifolia is. The ED, Hieracium pilosella, is consumed more frequently during the summer in EC. Of the GRA, Carex sp. is the species which differs more significantly, being more consumed during summer. In the natural pasture (MB), the life forms which are most commonly consumed are GR (50%), followed in importance by ND (23%), GRA (21%), considering the spring period. During the summer, the most consumed life form remains to be GRA (62%), followed in frequency of intake by GRA(17%), TS (12%) and in a lesser proportion ND (6%) (Figure 2). Comparing the two periods, we observe an increase in the intake of GRA and a decrease in the consumption of ND, which is accompanied by an increase in the frequency of consumption of woody plants (Figure 2). Within the GRA, the species most commonly consumed in the spring period is Poa sp., Elymus sp. next in importance. Deschampsia sp. has a frequency of intake of 1.7%. During the summer, Poa sp. and Festuca sp. are the most consumed, by a 22% and 29% respectively. Chilliotrichum sp. is more consumed in summer, compared to their intake during the spring. Since Cullen is an abandoned pasture in Ma Behety the frequency of intake of the invasive species (Hieracium) is low or null. In the summer period there is also a difference in the frequency of intake of GR, SR and TS, which is higher in MB. Taking into account the analysis of the principal components, 1 and 2 (75% of total variability), the diet of the sheep in EC during spring is differentiated from the rest of the diets, primarily because of the frequency in the intake of Phleum sp. y Cerastium sp. (Figure 3). The diets of summer in CU and in MB during both periods may be differentiated taking into consideration location and period, by the frequency of Festuca sp., Olsynium sp. and Chilliotrichum diffusum in take in the case of MB throughout all summer; while the same location during spring is characterized by the frequency of the intake of Carex sp. y Juncus sp.; and EC during the summer taking into account the Hiercium pilosella and Elymus agropyroides in take (Figure 3).
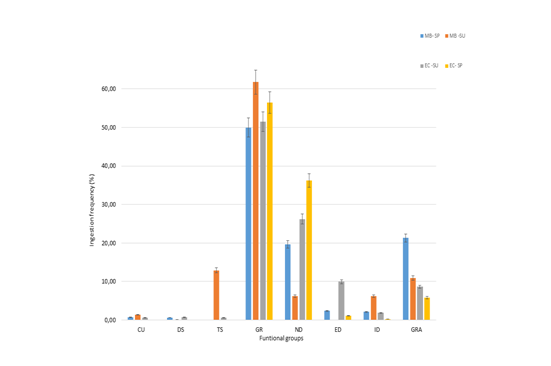
Figure 2 Frequency of the intake of different life forms, taking into account the ranch and period of time analyzed: CU, cushion; DS, dwarfshrub; TS, tallshrub; GR, grasses; ND, nativeherb dicotyledons; ED, exoticherb dicotyledons; ID, introducedherb dicotyledons to improve grasslands; GRA, graminoids; MB: Ma. Behety, EC: Cullen, SU: summer, SP: spring.
Differences found among diets not only reflect food offer but also may be associated to environmental conditions, location within the land and phenologic conditions of the plant species, all which may show the habitat use of sheep.
Other studies conducted throughout the continental extraandine Patagonia, in the steppe and in the Fueguian ecotone, describe similar changes also brought about overgrazing,51‒55 as well as a selectivity of diets associated to the location of the field and to the availability according to the ambient features regarding seasonality.56 It is common knowledge that associated to traditional handling; the formation of new habitats is brought about because of the action of sheep, which have increased the landscape’s diversity. Some of these habitats, such as the grasslands, the coironals and the open scrublands have improved their vitality and the chance to forage; while the Diddle-dee species have worsen it.57 The temperate semi-arid grasslands located in the central part of Argentina have a history of about 100 years of being grazed by sheep and cattle. In uplands, compositional changes associated with grazing include the replacement of palatable midgrasses for palatable shortgrasses.58,59 However, under conditions of long-term, heavy grazing, the palatable grasses commonly give way to an increase cover or even to an invasion by unpalatable grasses.60 The results presented by this study concur with what has been described by those authors, given that in the diet it was identified the intake of an invasive species, as Hieracium pilosella, whose presence is stronger in EC, an abandoned pasture where the species is taking over space.Herbivory influenced total vegetation cover but not species composition of plants colonising disturbed plots.This is reflected in the composition of the communities and of the studied diets.
This knowledge constitutes a basis for management and conservation of these natural grasslands in the Fueguian steppe. Management strategies should be targeted to the conservation of grasses which is the life form most susceptible to overgraze and cause disturbance. In addition, these results contribute to the state of progress of the invasive species Hieracium pilosella in the Fueguian steppe and to the knowledge of the incorporation of this species in the diet of livestock, and possibly in that of native herbivores.61‒63
We are in debt to Dr. J. Anchorena and Lic. C. Escartín for field assistance during the collection of material; to Mrs. María Dolores Montero for her collaboration in handling herbarium material; Estancia María Behety and Estancia Cullen to their owners and administrators, for their kindness.
Author declares that there is no conflict of interest.

©2018 Fernández, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.